Chlordecone
- CAS NO.:143-50-0
- Empirical Formula: C10Cl10O
- Molecular Weight: 490.64
- MDL number: MFCD00213544
- EINECS: 205-601-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-14 15:18:27
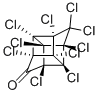
What is Chlordecone?
Description
Chemically, kepone is a chlorinated polycyclic insecticide and fungicide. Chlordecone was used as an insecticide for leaf-eating insects, ants, and cockroaches; as a larvicide for flies; and for control of insects that attack structures. Chlordecone was also used on bananas, nonbearing citrus trees, tobacco, ornamental shrubs, lawns, turf, and flowers. The dry powder is readily absorbed through the skin and respiratory tract. Occupational workers handling kepone without appropriate workplace safety dress and PPE suffered chemical poisoning. The symptoms included, tremors, jerky eye movements, memory loss, headaches, slurred speech, unsteadiness, lack of coordination, loss of weight, rash, enlarged liver, decreased libido, sterility, chest pain, and arthralgia (sharp pain, extending along a nerve or group of nerves, experienced in a joint and/or joints). Chlordecone was first introduced as a pesticide in 1958 and was used until 1978, when its use in the United States was discontinued (NCI 1976, IARC 1979, HSDB 2009).
Description
Chlordecone is an insecticide that was patented in 1952 by the now-defunct Allied Chemical Company. Millions of pounds were sold under the trade name Kepone, but it soon became clear that it is highly toxic to humans and wildlife.
Dumping chlordecone into the James River in Virginia caused 100 miles of the river to be closed to fishing for 13 years. Serious soil contamination by chlordecone occurred in the Caribbean islands of Martinique and Guadeloupe. In 2009, the Stockholm Convention banned its production and use worldwide.
Chemical properties
Kerosene is a white to pale yellow, flammable liquid that has wide use in household and industrial activities. For instance, in heating, as cooking fuel, in cleaning, degreasing, as a solvent, for paints, enamels, polishes, varnishes, and in asphalt coating.
Chemical properties
Kepone is a tan to white, odorless crystalline solid.
Physical properties
Colorless to tan, odorless, noncombustible, crystalline solid
The Uses of Chlordecone
Chlordecone, a chlorinated dicyclodiene compound, was introduced under the trade name Kepone in 1958. In the United States, Kepone found use as an ant and cockroach poison. Outside the United States, it was used primarily as a pesticide against the banana root borer. The compound is the ketone analog of mirex; it is a contaminant of mirex and is also a product of mirex degradation.
The Uses of Chlordecone
Formerly as insecticide, fungicide.
The Uses of Chlordecone
Insecticide; fungicide.
What are the applications of Application
Chlordecone is an organochlorine compound
Definition
ChEBI: An organochlorine compound with insecticidal activity.
General Description
Odorless colorless crystalline solid.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
A halogenated ketone. Ketones are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4.
Health Hazard
INHALATION AND INGESTION: These symptoms present in all affected patients - Neurologic Impairment - anxiety, irritability, memory disturbance, headache, tremors, opsiclonus, stuttering, slurred speech, and abnormal tandem gait.
Health Hazard
Kerosene toxicity is variable and is based on the composition. It is rapidly absorbed by the skin and accidental ingestion results in mucous membrane irritation, gastrointestinal irritation, vomiting, diarrhea, pneumonitis, CNS depression, drowsiness, coma, and may lead to death. Prolonged contact is also known to cause skin blisters and dermatitis. Studies with non-human primates have demonstrated that aerosols and kerosene aspiration into the lungs cause cellular damage. Household activities and possible long-term exposures to kerosine require proper care to avoid skin contact and possible damage. Kerosene should never be sucked by mouth.
Fire Hazard
Flash point data for KEPONE (TM) are not available; however, KEPONE (TM) is probably combustible.
Potential Exposure
Kepone was registered for the control of rootborers on bananas with a residue tolerance of 0.01 ppm. This constituted the only food or feed use of Kepone. Nonfood uses included wireworm control in tobacco fields and bait to control ants and other insects in indoor and outdoor areas. A rebuttable presumption against registration of chlordecone was issued by the United States Environmental Protection Agency on March 25, 1976 on the basis of oncogenicity. The trademarked Kepone and products of six formulations were the subject of voluntary cancellation according to a United States Environmental Protection Agency notice dated July 27, 1977. In a series of decisions, the first of which was issued on June 17, 1976, the EPA effectively canceled all registered products containing Kepone as of May 1, 1978.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Speed in removing material from skinis of extreme importance. Shampoo hair promptly if contaminated. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, beginrescue breathing (using universal precautions, includingresuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Qualified medical personne may consider the administrationof cholestyramine resin (QUESTRAN). Medical personnelshould wear Neoprene? gloves as protection against contamination (Dreisbach).
Carcinogenicity
Kepone (chlordecone) is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Environmental Fate
Photolytic. Kepone-contaminated soils from a site in Hopewell, VA were analyzed by
GC/MS. 8-Chloro and 9-chloro homologs identified suggested these were photodegradation
products of kepone (Borsetti and Roach, 1978). Products identified from the photolysis
of kepone in cyclohexane were 1,2,3,4,6,7,9,10,10-nonachloro-5,5-dihydroxypentacyclo[
5.3.0.02,6.03,9.04,8]decane for the hydrate and 1,2,3,4,6,7,9,10,10-nonachloro-5,5-
dimethoxypentacyclo[5.3.0.02,6.03,9.04,8]decane (Alley et al., 1974).
Chemical/Physical. Readily reacts with moisture forming hydrates (Hollifield, 1979).
Decomposes at 350°C (Windholz et al., 1983) probably emitting toxic chlorine fumes.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with Keponeyou should be trained on its proper handling and storage. Aregulated, marked area should be established where thischemical is handled, used, or stored in compliance withOSHA Standard 1910.1045. Store in a cool, dry place in arefrigerator under inert atmosphere. Keep away fromacids and acid fumes.
Shipping
UN2761 Organochlorine pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Acids, acid fumes.
Waste Disposal
A process has been developed which effects chlordecone degradation by treatment of aqueous wastes with UV radiation in the presence of hydrogen in aqueous sodium hydroxide solution. Up to 95% decomposition was effected by this process. Chlordecone previously presented serious disposal problems because of its great resistance to bio- and photo degradation in the environment. It is highly toxic to normally occur in degrading microorganisms. Although it can undergo some photodecomposition when exposed to sunlight to the dihydro compound (leaving a compound with 8 chloro substituents) that degradation product does not significantly reduce toxicity. Disposal by incineration with HCl scrubbing is recommended.
Properties of Chlordecone
| Melting point: | 350℃ (DEC.) |
| Boiling point: | 450.5±45.0 °C(Predicted) |
| Density | 2.27 |
| vapor pressure | 2.25 x 10-7 mmHg at 25 °C (Kilzer et al., 1979) |
| Flash point: | >100 °C |
| storage temp. | 0-6°C |
| solubility | Soluble in acetic acid, alcohols, and ketones (Windholz et al., 1983) |
| form | Tan to white crystalline solid |
| Water Solubility | (mg/L): 7.600 at 24 °C (shake flask-nephelometry, Hollifield, 1979) 2.7 at 20–25 °C (Kilzer et al., 1979) |
| Merck | 13,2098 |
| Henry's Law Constant | (x 10-2 atm?m3/mol):
3.11 at 25 °C (approximate - calculated from experimentally determined water solubility and
vapor pressure values) |
| Exposure limits | NIOSH REL: TWA 1 mg/m3. |
| IARC | 2B (Vol. 20, Sup 7) 1987 |
| EPA Substance Registry System | Chlordecone (143-50-0) |
Safety information for Chlordecone
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H311:Acute toxicity,dermal H351:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P312:Call a POISON CENTER or doctor/physician if you feel unwell. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P501:Dispose of contents/container to..… |
Computed Descriptors for Chlordecone
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 Chlordecone CAS 143-50-0View Details
Chlordecone CAS 143-50-0View Details
143-50-0 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4