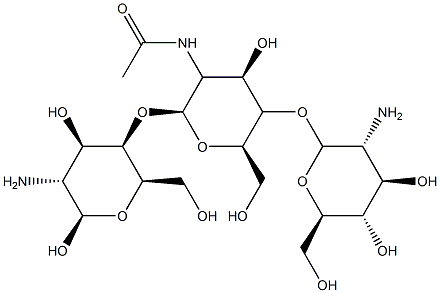
Chitosan
Synonym(s):2-Amino-2-deoxy-(1→4)-β-D -glucopyranan;Deacetylated chitin;Poly(D -glucosamine);Poly-(1,4-β-D -glucopyranosamine)
- CAS NO.:9012-76-4
- Empirical Formula: C6H11NO4X2
- Molecular Weight: 161.16
- MDL number: MFCD00161512
- EINECS: 618-480-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-21 19:13:37

What is Chitosan?
Chemical properties
Chitosan occurs as odorless, white or creamy-white powder or flakes. Fiber formation is quite common during precipitation and the chitosan may look ‘cottonlike’.
Chemical properties
Chitosan is a weak base and is insoluble in water and organic solvent. However, it is soluble in dilute aqueous acidic solution (pH<6.5), which can convert glucosamine units into soluble form R – NH 3 +.
Occurrence
Chitosan comes from the shell of marine crustaceans.
The Uses of Chitosan
Forms gels with multivalent anions. Gives clear solutions that dry to strong, clear films.Flocculant, protein precipitation, encapsulating agent and aqueous thickener.
Chitosan is a natural polycationic linear polysaccharide derived from chitin. The low solubility of chitosan in neutral and alkaline solution limits its application.
Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications
The Uses of Chitosan
Ideal for wound healing and hemostasis; biosurgery and ophthalmology; scaffold and cell therapy; and drug delivery and vaccines
The Uses of Chitosan
chitosan is a film-forming polysaccharide that can aid the skin’s moisture content and moisture-retention capacity by preventing transepidermal water loss. In addition, it appears to help bind other ingredients (for example, to increase liposome stability), thereby increasing the availability of active ingredients to the skin. Anti-bacterial properties are also associated with chitosan, as well as an ability to enhance the microbiological stability of a preparation. Some studies show that it can help improve the water-resistance properties of sun protection creams and lotions, and the longevity of a fragrance’s scent (the perfume adheres more strongly to the skin and evaporates more slowly, over a longer period). It acts as a skin conditioner, improving skin softness and suppleness. It may be found in moisturizers, sunscreens, and acne preparations, in addition to hair care products. It is the decarboxylated form of chitin and can hold water without creating a feeling of tackiness in a cosmetic preparation. According to some chitosan suppliers, the use of shrimp shells is one of the most important sources. They consist of 30–35 percent protein, 30–35 percent minerals, and 15–20 percent chitin, with some traces of lipids, dyes, and soluble proteins.
What are the applications of Application
Chitosan is a naturally occurring biocompatible, antibacterial polyelectrolyte biomaterial
Production Methods
Chitosan is manufactured commercially by chemically treating the shells of crustaceans such as shrimps and crabs. The basic manufacturing process involves the removal of proteins by treatment with alkali and of minerals such as calcium carbonate and calcium phosphate by treatment with acid. Before these treatments, the shells are ground to make them more accessible. The shells are initially deproteinized by treatment with an aqueous sodium hydroxide 3–5% solution. The resulting product is neutralized and calcium is removed by treatment with an aqueous hydrochloric acid 3–5% solution at room temperature to precipitate chitin. The chitin is dried so that it can be stored as a stable intermediate for deacetylation to chitosan at a later stage. NDeacetylation of chitin is achieved by treatment with an aqueous sodium hydroxide 40–45% solution at elevated temperature (1108℃), and the precipitate is washed with water. The crude sample is dissolved in acetic acid 2% and the insoluble material is removed. The resulting clear supernatant solution is neutralized with aqueous sodium hydroxide solution to give a purified white precipitate of chitosan. The product can then be further purified and ground to a fine uniform powder or granules. The animals from which chitosan is derived must fulfil the requirements for the health of animals suitable for human consumption to the satisfaction of the competent authority. The method of production must consider inactivation or removal of any contamination by viruses or other infectious agents.
Benefits
Chitosan is a fibrous substance that might reduce how much fat and cholesterol the body absorbs from foods. It also helps blood clot when applied to wounds.
General Description
Chitosan is a linear amino polysaccharide composed of approximately 20% β1,4-linked N-acetyl-D-glucosamine (GlcNAc) and approximately 80% β1,4-linked D-glucosamine (GlcN) that is prepared by the partial deacetylation of chitin in hot alkali.
Pharmaceutical Applications
Chitosan is used in cosmetics and is under investigation for use in a number of pharmaceutical formulations. The suitability and performance of chitosan as a component of pharmaceutical formulations for drug delivery applications has been investigated in numerous studies. These include controlled drug delivery applications, use as a component of mucoadhesive dosage forms, rapid release dosage forms, improved peptide delivery, colonic drug delivery systems, and use for gene delivery. Chitosan has been processed into several pharmaceutical forms including gels, films, beads, microspheres, tablets, and coatings for liposomes. Furthermore, chitosan may be processed into drug delivery systems using several techniques including spray-drying, coacervation, direct compression, and conventional granulation processes.
Safety
Chitosan is being investigated widely for use as an excipient in oral
and other pharmaceutical formulations. It is also used in cosmetics.
Chitosan is generally regarded as a nontoxic and nonirritant
material. It is biocompatible with both healthy and infected
skin. Chitosan has been shown to be biodegradable.
LD50 (mouse, oral): >16 g/kg
storage
Chitosan powder is a stable material at room temperature, although it is hygroscopic after drying. Chitosan should be stored in a tightly closed container in a cool, dry place. The PhEur 6.5 specifies that chitosan should be stored at a temperature of 2–88℃.
Incompatibilities
Chitosan is incompatible with strong oxidizing agents.
Regulatory Status
Chitosan is registered as a food supplement in some countries.
Properties of Chitosan
| Melting point: | 102.5 °C |
| Density | 1 g/cm3 |
| storage temp. | 2-8°C |
| solubility | dilute aqueous acid (pH <6.5).: soluble |
| form | (Coarse ground flakes and powder) |
| color | White to Off-white |
| Odor | Odorless |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 9012-76-4 |
| EPA Substance Registry System | Chitosan (9012-76-4) |
Safety information for Chitosan
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Chitosan
Chitosan manufacturer
CEFA CILINAS BIOTICS PVT LTD
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran





You may like
-
 9012-76-4 Chitosan 98%View Details
9012-76-4 Chitosan 98%View Details
9012-76-4 -
 Chitosan 9012-76-4 99%View Details
Chitosan 9012-76-4 99%View Details
9012-76-4 -
 Chitosan Nanopowder CAS 9012-76-4View Details
Chitosan Nanopowder CAS 9012-76-4View Details
9012-76-4 -
 Chitosan (High MW) extrapure CAS 9012-76-4View Details
Chitosan (High MW) extrapure CAS 9012-76-4View Details
9012-76-4 -
 Chitosan, medium molecular weight CAS 9012-76-4View Details
Chitosan, medium molecular weight CAS 9012-76-4View Details
9012-76-4 -
 Chitosan CAS 9012-76-4View Details
Chitosan CAS 9012-76-4View Details
9012-76-4 -
 Chitosan Crystal CAS 9012-76-4View Details
Chitosan Crystal CAS 9012-76-4View Details
9012-76-4 -
 Chitosan powder CASView Details
Chitosan powder CASView Details
