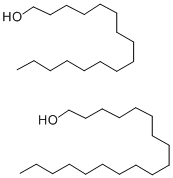
CETEARYL ALCOHOL
- CAS NO.:8005-44-5
- Empirical Formula: C16H34O
- Molecular Weight: 242.440002441406
- MDL number: MFCD04113584
- EINECS: 267-008-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-06 18:33:45
What is CETEARYL ALCOHOL?
Chemical properties
Cetostearyl alcohol occurs as white or cream-colored unctuous masses, flakes, pellets or granules. It has a faint, characteristic sweet odor. On heating, cetostearyl alcohol melts to a clear, colorless or pale yellow-colored liquid free of suspended matter.
Production Methods
Cetostearyl alcohol is prepared by the reduction of the appropriate fatty acids from vegetable and animal sources. Cetostearyl alcohol can also be prepared directly from hydrocarbon sources.
Pharmaceutical Applications
Cetostearyl alcohol is used in cosmetics and topical pharmaceutical preparations. In topical pharmaceutical formulations, cetostearyl alcohol will increase the viscosity and act as an emulsifier in both water-in-oil and oil-in-water emulsions. Cetostearyl alcohol will stablize an emulsion and also act as a co-emulsifier, thus decreasing the total amount of surfactant required to form a stable emulsion. Cetostearyl alcohol is also used in the preparation of nonaqueous creams and sticks, and in nonlathering shaving creams. Research articles have been published in which cetostearyl alcohol has been used to control or slow the dissolution rate of tablets or microspheres containing water-soluble drugs, or poorly watersoluble drugs, as well as to stabilize amorphous systems. In combination with other surfactants, cetostearyl alcohol forms emulsions with very complex microstructures. These microstructures can include liquid crystals, lamellar structures, and gel phases.
Safety
Cetostearyl alcohol is mainly used in topical pharmaceutical
formulations and topical cosmetic formulations.
Cetostearyl alcohol is generally regarded as a nontoxic
material. Although it is essentially nonirritating, sensitization
reactions to cetostearyl, cetyl, and stearyl alcohols have been
reported.
Gamma radiation has been shown to be feasible for sterilization
of petrolatum containing cetostearyl alcohol resulting in low levels
of radiolysis products, which are of low toxicity.
storage
Cetostearyl alcohol is stable under normal storage conditions. Cetostearyl alcohol should be stored in a well-closed container in a cool, dry place.
Incompatibilities
Incompatible with strong oxidizing agents and metal salts.
Regulatory Status
Accepted as an indirect food additive and as an adhesive and a component of packaging coatings in the USA. Included in the FDA Inactive Ingredients Database (oral tablets; topical emulsions, lotions, ointments; vaginal suppositories). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of CETEARYL ALCOHOL
| solubility | Soluble in ethanol (95%), ether, and oil; practically
insoluble in water. |
| InChI | InChI=1S/C18H38O.C16H34O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19;1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17/h19H,2-18H2,1H3;17H,2-16H2,1H3 |
| CAS DataBase Reference | 8005-44-5(CAS DataBase Reference) |
Safety information for CETEARYL ALCOHOL
Computed Descriptors for CETEARYL ALCOHOL
| InChIKey | UBHWBODXJBSFLH-UHFFFAOYSA-N |
| SMILES | C(O)CCCCCCCCCCCCCCC.C(O)CCCCCCCCCCCCCCCCC |
Abamectin manufacturer
Anand Agencies
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

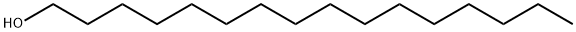


You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4