GADOLINIUM
Synonym(s):Gadolinium;
- CAS NO.:7440-54-2
- Empirical Formula: Gd
- Molecular Weight: 157.25
- MDL number: MFCD00011022
- EINECS: 231-162-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
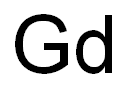
What is GADOLINIUM?
Chemical properties
metal foil, chunks or powder. The powder of gadolinium is highly flammable; incompatible with strong oxidising agents, halogens, acids; and reacts with water or moisture.
Physical properties
Gadolinium is silvery-white, soft, malleable, and ductile with a metallic luster. It is the secondof what is referred to as the dysprosium, subgroup in the middle of the lanthanide seriesof rare-earths. It tarnishes in air, forming the oxide (Gd2O3) on the surface, which flakes offthe surface, exposing a fresh metal that in turn oxidizes.
Its melting point is 1,313°C, its boiling point is 3,273°C, and its density is 7.90g/cm3.
Isotopes
There are 39 isotopes of gadolinium. Seven of these are stable. They are: Gd-54, which makes up 2.18% of all the gadolinium found in the Earth’s crust; Gd-55,supplying 14.80%; Gd-156, making up 20.47%; Gd-157, constituting 15.56%; and Gd-158, accounting for 24.85%. In addition, there are two isotopes of gadolinium that areradioactive and with such long half-lives that they still exist in the Earth’s crust. They areregarded as stable isotopes along with the other seven. They are Gd-152 (1.08×10+14years), which exists in just 0.20% in abundance, and Gd-160 (1.3×10+21 years), foundin 21.86% abundance.
Origin of Name
Named for the mineral gadolinite, which was named for the French chemist Johann Gadolin.
Occurrence
Gadolinium is the 40th most abundant element on Earth and the sixth most abundant ofthe rare-earths found in the Earth’s crust (6.4 ppm). Like many other rare-earths, gadoliniumis found in monazite river sand in India and Brazil and the beach sand of Florida as well asin bastnasite ores in southern California. Similar to other rare-earths, gadolinium is recoveredfrom its minerals by the ion-exchange process. It is also produced by nuclear fission in atomicreactors designed to produce electricity.
History
Gadolinia, the oxide of gadolinium, was separated by Marignac in 1880 and Lecoq de Boisbaudran independently isolated Gadolinium from Mosander’s “yttria” in 1886. The element was named for the mineral gadolinite from which this rare earth was originally obtained. Gadolinium is found in several other minerals, including monazite and bastnasite, which are of commercial importance. With the development of ion-exchange and solvent extraction techniques, the availability and price of gadolinium and the other rare-earth metals have greatly improved. Thirtyone isotopes and isomers of gadolinium are now recognized; seven are stable and occur naturally. The metal can be prepared by the reduction of the anhydrous fluoride with metallic calcium. As with other related rare-earth metals, it is silvery white, has a metallic luster, and is malleable and ductile. At room temperature, gadolinium crystallizes in the hexagonal, close-packed α form. Upon heating to 1235°C, α gadolinium transforms into the β form, which has a body-centered cubic structure. The metal is relatively stable in dry air, but in moist air it tarnishes with the formation of a loosely adhering oxide film which splits off and exposes more surface to oxidation. The metal reacts slowly with water and is soluble in dilute acid. Gadolinium has the highest thermal neutron capture cross-section of any known element (49,000 barns). Natural gadolinium is a mixture of seven isotopes. Two of these, 155Gd and 157Gd, have excellent capture characteristics, but they are present naturally in low concentrations. As a result, gadolinium has a very fast burnout rate and has limited use as a nuclear control rod material. It has been used in making gadolinium yttrium garnets, which have microwave applications. Compounds of gadolinium are used in making phosphors for color TV tubes. The metal has unusual superconductive properties. As little as 1% gadolinium has been found to improve the workability and resistance of iron, chromium, and related alloys to high temperatures and oxidation. Gadolinium ethyl sulfate has extremely low noise characteristics and may find use in duplicating the performance of amplifiers, such as the maser. The metal is ferromagnetic. Gadolinium is unique for its high magnetic moment and for its special Curie temperature (above which ferromagnetism vanishes) lying just at room temperature. This suggests uses as a magnetic component that senses hot and cold. The price of the metal is about $5/g (99.9% purity).
Characteristics
Gadolinium, unlike most of the rare earths in the dysprosium subgroup, reacts slowlywith water, releasing hydrogen. It is strongly magnetic at low temperatures. Two of its stableisotopes (Gd-155 and Gd-157) have the greatest ability of all natural elements to absorb thermalneutrons to control the fission chain reaction in nuclear reactors. However, few of theseisotopes are found in the ores of gadolinium.
The Uses of GADOLINIUM
Gadolinium’s main use is based on its ability to absorb neutrons, thus making it ideal as aneutron-shielding and neutron-absorbing metal. It is also used as an alloying agent for steel andother metals to make the metals more workable and to be able to withstand low temperatures.
Gadolinium is used in the manufacture of electronics and can be combined with yttriumto make garnets used in microwaves. Gadolinium is used as a catalyst to speed up chemicalreactions, and to activate phosphor compounds in TV screens and cast filaments in electricaldevices. It is also used in high-temperature furnaces. Gadolinium is paramagnetic at normalroom temperatures (weaker than ferromagnetic) and becomes strongly ferromagnetic at verycold temperatures.
The Uses of GADOLINIUM
Neutron shielding, garnets in microwave filters, phosphor activator, catalyst, scavenger for oxygen in titanium production
The Uses of GADOLINIUM
Oxide of Gadolinium foil is used in the control rods of some nuclear reactors. It is used as nuclear control rod material. Neutron shielding, phosphor activator, catalyst, scavenger for oxygen in titanium production. A metal element that is used in magnetic resonance imaging (MRI) and other imaging methods. It has been used in making gadolinium yttrium garnets, which have microwave applications.
Background
Gadolinium is under investigation in Hypertension, ACUTE KIDNEY INJURY, and Chronic Kidney Disease. Gadolinium has been investigated for the basic science of CAD, Multiple Sclerosis, and Coronary Artery Disease.
Definition
A rare-earth element of the lanthanide series, atomic number 64, group IIIB of the periodic table, aw 157.25, valence of 3; seven natural isotopes.
Definition
A ductile malleable silvery element of the lanthanoid series of metals. It occurs in association with other lanthanoids. Gadolinium is used in alloys, magnets, and in the electronics industry. Symbol: Gd; m.p. 1313°C; b.p. 3266°C; r.d. 7.9 (25°C); p.n. 64; r.a.m. 157.25.
Definition
gadolinium: Symbol Gd. A soft silverymetallic element belonging tothe lanthanoids; a.n. 64; r.a.m.157.25; r.d. 7.901 (20°C); m.p. 1313°C;b.p. 3266°C. It occurs in gadolinite,xenotime, monazite, and residuesfrom uranium ores. There are sevenstable natural isotopes and elevenartificial isotopes are known. Two ofthe natural isotopes, gadolinium–155and gadolinium–157, are the bestneutron absorbers of all the elements.The metal has found limitedapplications in nuclear technologyand in ferromagnetic alloys (withcobalt, copper, iron, and cerium).Gadolinium compounds are used inelectronic components. The elementwas discovered by Jean de Marignac(1817–94) in 1880.
Hazard
The halogens of gadolinium are very toxic, and gadolinium nitrate is explosive. As withmost rare-earths, care should be taken not to inhale fumes or ingest particles of gadolinium.
Flammability and Explosibility
Flammable
Metabolism
Not Available
Properties of GADOLINIUM
| Melting point: | 1313 °C (lit.) |
| Boiling point: | 3273 °C (lit.) |
| Density | 7.886 g/mL at 25 °C (lit.) |
| Flash point: | 3266°C |
| solubility | soluble in dilute acid solutions |
| form | ingot |
| color | Silver |
| Specific Gravity | 7.898 |
| Resistivity | 126 μΩ-cm, 20°C |
| Water Solubility | Soluble in magnesium and diluted acid. Insoluble in water. |
| Sensitive | Air & Moisture Sensitive |
| Merck | 13,4347 |
| Exposure limits | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| Stability: | Stable. Powder is highly flammable. Incompatible with strong oxiziding agents, halogens, acids. Powder may react with water or moisture. |
| CAS DataBase Reference | 7440-54-2(CAS DataBase Reference) |
| EPA Substance Registry System | Gadolinium (7440-54-2) |
Safety information for GADOLINIUM
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H228:Flammable solids H261:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P370+P378:In case of fire: Use … for extinction. |
Computed Descriptors for GADOLINIUM
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

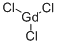

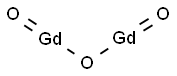

You may like
-
 Gadolinium, Rare Earth Oxides Content CAS 7440-54-2View Details
Gadolinium, Rare Earth Oxides Content CAS 7440-54-2View Details
7440-54-2 -
 Gadolinium pieces CAS 7440-54-2View Details
Gadolinium pieces CAS 7440-54-2View Details
7440-54-2 -
 Gadolinium rod, 6.35mm (0.25 in.) dia. CAS 7440-54-2View Details
Gadolinium rod, 6.35mm (0.25 in.) dia. CAS 7440-54-2View Details
7440-54-2 -
 Gadolinium rod, 6.35mm (0.25 in.) dia. CAS 7440-54-2View Details
Gadolinium rod, 6.35mm (0.25 in.) dia. CAS 7440-54-2View Details
7440-54-2 -
 Gadolinium rod, 6.35mm (0.25 in.) dia. CAS 7440-54-2View Details
Gadolinium rod, 6.35mm (0.25 in.) dia. CAS 7440-54-2View Details
7440-54-2 -
 Gadolinium rod, 12.7mm (0.5 in.) dia. CAS 7440-54-2View Details
Gadolinium rod, 12.7mm (0.5 in.) dia. CAS 7440-54-2View Details
7440-54-2 -
 Gadolinium rod, 12.7mm (0.5 in.) dia. CAS 7440-54-2View Details
Gadolinium rod, 12.7mm (0.5 in.) dia. CAS 7440-54-2View Details
7440-54-2 -
 Gadolinium powder CAS 7440-54-2View Details
Gadolinium powder CAS 7440-54-2View Details
7440-54-2