CASTANOSPERMINE
Synonym(s):Castanospermine;(1S,6S,7R,8R,8aR)-Octahydro-1,6,7,8-indolizinetetrol;(1S,6S,7R,8aR)-Tetrahydroxyoctahydroindolizine;1,6,7,8-Tetrahydroxyoctahydroindolizine;(1S,6S,7R,8R,8aR)-1,6,7,8-Tetrahydroxyoctahydroindolizidine
- CAS NO.:79831-76-8
- Empirical Formula: C8H15NO4
- Molecular Weight: 189.21
- MDL number: MFCD00017555
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
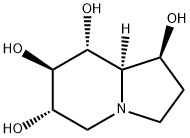
What is CASTANOSPERMINE?
Description
Glucosidases catalyze the cleavage of individual glucosyl residues from various glycoconjugates, including complex carbohydrates and glycoproteins. Castanospermine is an inhibitor of both α-
Chemical properties
White Crystalline Solid
Occurrence
The seeds of Castanospermum australe yield this simple alkaloid. It forms colourless crystals from EtOH and is dextrorotatory with a specific rotation of [α]D25 +79.7° (c 0.93, H20). Castano spermine has been characterized as the crystalline methiodide, m.p. 11O-112°C.
The Uses of CASTANOSPERMINE
Castanospermine is a plant alkaloid shown to be a potent inhibitor of lysosomal a-or beta- glucosidase. It also inhibits mammalian glucosidase 1 and beta-mannosidase from sweet almonds and fungal beta-xylosidase.
The Uses of CASTANOSPERMINE
a-L-fucosidases inhibitor
The Uses of CASTANOSPERMINE
Castanospermine, is used as a glycosidase inhibitor and antiinflammatory agent.castanospermine is used to inhibit syncytium formation between HIV-infected and CD4-expressing cells and may also interfere with infectivity. It has also been demonstrated that this agent inhibits inflammation at the level of leukocyte extravasation in rat models of experimental adjuvant-induced arthritis and autoimmune encephalomyelitis. Castanospermine is an inhibitor of Glucosidase I, Maltase-glucoamylase, and MANBA. Potent inhibitor of α- and β-glucosidases, especially glucosidase l (required for glucoprotein processing by transfer of mannose and glucose from asparagine-linked lipids). Inhibits HIV syncytium formation and replication.
What are the applications of Application
Castanospermine is a glycosidase inhibitor and antiinflammatory agent
Definition
ChEBI: Castanospermine is a tetrahydroxyindolizidine alkaloid that consists of octahydroindolizine having four hydroxy substituents located at positions 1, 6, 7 and 8 (the 1S,6S,7R,8R,8aR-diastereomer). It has a role as a metabolite, an anti-HIV-1 agent, an anti-inflammatory agent and an EC 3.2.1.* (glycosidase) inhibitor.
Biological Activity
Potent inhibitor of α - and β -glucosidases, especially glucosidase l (required for glucoprotein processing by transfer of mannose and glucose from asparagine-linked lipids). Inhibits HIV syncytium formation and replication.
storage
Store at RT
References
1) Saul et al. (1984), Studies on the mechanism of castanospermine inhibition of alpha- and beta-glucosidases; Arch. Biochem. Biophys, 230 668 2) Repp et al. (1985), The effects of processing inhibitors on N-linked oligosaccharides on the intracellular migration of glycoprotein E2 of mouse hepatitis virus and the maturation of coronavirus particles; J. Biol. Chem., 260 15873 3) Gruters et al. (1987), Interference with HIV-induced syncytium formation and viral infectivity by inhibitors of trimming glucosidase; Nature, 330 74 4) Franc et al. (1990), Effects of deoxymannojirimycin and castanospermine on the polarized secretion of thyroglobulin; Endocrinology, 126 1464 5) Pili et al. (1995), The alpha-glucosidase I inhibitor castanospermine alters endothelial cell glycosylation, prevents angiogenesis, and inhibits tumor growth; Mol. Cancer Res., 55 2920 6) Rhinehart et al. (1987), Castanospermine blocks the hyperglycemic response to carbohydrates in vivo: a result of intestinal disaccharidase inhibition; Life Sci., 41 2325
Properties of CASTANOSPERMINE
| Melting point: | 213-217 °C (lit.) |
| Boiling point: | 421.9±45.0 °C(Predicted) |
| alpha | D25 +79.7° (c = 0.93 in water) |
| Density | 1.53±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | 1 M HCl: 20 mg/mL, clear, very faintly yellow |
| form | White solid |
| pka | 6.09(at 25℃) |
| color | White or off-white |
| optical activity | [α]20/D +80°, c = 0.9 in H2O |
| Water Solubility | Soluble in water |
| Merck | 13,1906 |
| BRN | 3588654 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in distilled water may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 79831-76-8(CAS DataBase Reference) |
Safety information for CASTANOSPERMINE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H312:Acute toxicity,dermal H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P321:Specific treatment (see … on this label). P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. |
Computed Descriptors for CASTANOSPERMINE
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Castanospermine 95% CAS 79831-76-8View Details
Castanospermine 95% CAS 79831-76-8View Details
79831-76-8 -
 Castanospermine, Castanospermum australe CAS 79831-76-8View Details
Castanospermine, Castanospermum australe CAS 79831-76-8View Details
79831-76-8 -
 (1S,6S,7R,8R,8aR)-1,6,7,8-Tetrahydroxyindolizidine CAS 79831-76-8View Details
(1S,6S,7R,8R,8aR)-1,6,7,8-Tetrahydroxyindolizidine CAS 79831-76-8View Details
79831-76-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8