Carteolol hydrochloride
Synonym(s):Carteolol monohydrochloride
- CAS NO.:51781-21-6
- Empirical Formula: C16H25ClN2O3
- Molecular Weight: 328.83
- MDL number: MFCD00941499
- EINECS: 257-415-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-29 15:43:00
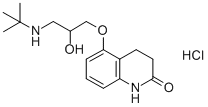
What is Carteolol hydrochloride?
Chemical properties
Crystalline Solid
The Uses of Carteolol hydrochloride
-Adrenergic blocker. Antihypertensive; antianginal; antiarrhythmic; antiglaucoma
The Uses of Carteolol hydrochloride
β-Adrenergic blocker. Antihypertensive; antianginal; antiarrhythmic; antiglaucoma.
What are the applications of Application
Carteolol Hydrochloride is A β-Adrenergic blocker
brand name
Cartrol (Abbott); Ocupress (Novartis).
Biological Activity
beta-adrenergic antagonist are an important class of drugs for the treatment of various heart diseases, such as high blood pressure, insufficiency of blood flow to the heart muscle, irregular heart beat, thickened heart muscle, and decreased ability of the heart to empty or fill normally. β-blockers can also be used to treat migraine headache and increased eye pressure (glaucoma). carteolol is a nonselective beta-adrenoceptor antagonist.
Pharmacology
Carteolol hydrochloride is a powerful beta-adrenergic receptor antagonist characterized by a long-term pharmacological action, the lack of beta receptor selectivity, an intrinsic sympathomimetic activity (ISA), and a slight local anaesthetic activity (approximately 1/10 of that of propranolol). This drug has a dose-dependent hypotensive effect and does not engender the onset of tachyphylaxis. The pharmacological action consists in the decreased formation of aqueous humor, probably by acting at the level of the ciliary body presenting a wealth of ?2 receptors. The intrinsic sympathomimetic activity of carteolol is responsible for the low incidence of side effects and, thus, for maintaining the heart rate, pulse rate, and arterial blood pressure within the normal physiological range.
Pharmacokinetics
The instillation of carteolol hydrochloride is followed by the rapid absorption by the ocular tissues. Following the instillation of 10μl of 14C-carteolol ophthalmic solution 20 mg/ml into rabbit eyes, the highest platelet and tissue concentrations were obtained between 30 minutes to 2 hours. The study shows that, following topical application, carteolol spreads rapidly into the ocular tissues, passing the corneal barrier. The highest concentrations of 14C-carteolol were found in the following ocular tissues: cornea, iris, anterior sclera, ciliary body conjunctiva, and extraocular muscle. Slightly lower concentrations were found in the retina, choroid, posterior sclera, optic nerve, and aqueous humor. The concentration was especially low in the lens, vitreous body, and plasma. In the various tissues of the contralateral eye, very low radioactivity levels were measured. After the instillation of one drop of carteolol ophthalmic solution 20 mg/ml in healthy volunteers, the plasma concentrations were negligible (1-2 ng/ml). Approximately 16% of the dose is excreted in the urine as an unchanged compound during the first 24 hours after the instillation of a single drop of carteolol hydrochloride 20 mg/ml. The urinary elimination half-life following topical application is about 5 hours.
Clinical Use
The chemical name for carteolol hydrochloride (carteolol) is (±)-5-[3-[(1,1-dimethyl ethyl) amino]-2-hydroxypropoxy]-3,4-dihydro-2(1H)-quinolinone monohydrochloride. Carteolol Hydrochloride (carteolol) Ophthalmic Solution USP, 1% is a nonselective beta-adrenoceptor blocking agent for ophthalmic use. Each mL of sterile solution contains Active: carteolol hydrochloride (carteolol) 10 mg (1%). This drug is used to treat open-angle glaucoma and other causes of high pressure inside the eye. Carteolol hydrochloride ophthalmic may interact with oral carteolol, digoxin, reserpine, insulin or oral diabetes medications, other beta-blockers, calcium channel blockers, or medicines to treat psychiatric disorders.
in vitro
previous findings demonstrate that carteolol, unlike xamoterol, is a nonconventional partial agonist, which causes agonistic effects via interaction with the low-affinity propranolol-resistant site of β1-adrenoceptors and antagonistic actions by the high-affinity site of the same receptors [1].
in vivo
both 8-oh carteolol and carteolol suppressed the water-load induced intraocular pressure (iop) rise in rabbits. 8-oh carteolol was more effective in suppressing water-load induced iop rise in rabbits compared with carteolol on equimolar basis. both 8-oh carteololand carteolol caused a significant decrease in iop in monkeys. 8-oh carteolol was estimated to be more potent than carteolol in lowering iop on equimolar basis in monkeys [2].
References
[1] maura floreani, guglielmina froldi, luigi quintieri, katia varani, pier andrea borea, maria teresa dorigo and paola dorigo. in vitro evidence that carteolol is a nonconventional partial agonist of guinea pig cardiac β1-adrenoceptors: a comparison with xamoterol. jpet 315:1386–1395, 2005
[2] sugiyama k, enya t, kitazawa y. ocular hypotensive effect of 8-hydroxycarteolol, a metabolite of carteolol. int ophthalmol. 1989 jan;13(1-2):85-9.
[3] trinquand c, romanet jp, nordmann jp, allaire c; groupe d'étude. efficacy and safety of long-acting carteolol 1% once daily. a double-masked, randomized study. j fr ophtalmol. 2003 feb;26(2):131-6.
Properties of Carteolol hydrochloride
| Melting point: | 278°C |
| storage temp. | Refrigerator |
| solubility | Soluble in water, sparingly soluble in methanol, slightly soluble in ethanol 96 per cent, practically insoluble in methylene chloride. |
| form | neat |
| form | Solid |
| color | White to Off-White |
| Merck | 14,1866 |
Safety information for Carteolol hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Carteolol hydrochloride
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

![N1-[3-(3-AMINOPROPOXY)PHENYL]ACETAMIDE](https://img.chemicalbook.in/CAS/GIF/189683-22-5.gif)
You may like
-
 51781-21-6 Carteolol Hydrochloride 99%View Details
51781-21-6 Carteolol Hydrochloride 99%View Details
51781-21-6 -
 Carteolol hydrochloride 98%View Details
Carteolol hydrochloride 98%View Details -
 Carteolol hydrochloride 95% CAS 51781-21-6View Details
Carteolol hydrochloride 95% CAS 51781-21-6View Details
51781-21-6 -
 Carteolol hydrochloride CAS 51781-21-6View Details
Carteolol hydrochloride CAS 51781-21-6View Details
51781-21-6 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
