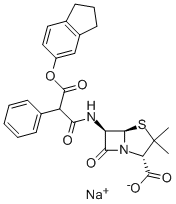
CARINDACILLIN
- CAS NO.:35531-88-5
- Empirical Formula: C26H26N2O6S
- Molecular Weight: 494.56
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:38
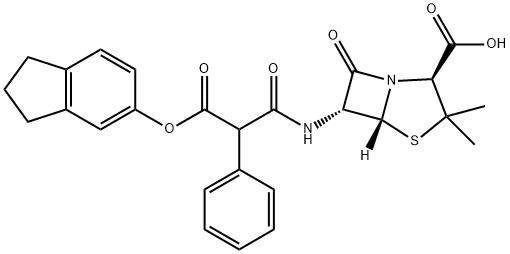
What is CARINDACILLIN?
Originator
Geocillin,Roerig,US,1972
The Uses of CARINDACILLIN
Antibacterial.
Background
Carindacillin or Carbenicillin isdanyl was an oral penicillin prodrug of carbenicillin marketed by Pfizer as Geocillin. It is no longer marketed in the United States.
Indications
For the treatment of acute and chronic infections of the upper and lower urinary tract and in asymptomatic bacteriuria (due to susceptible strains Escherichia coli, Proteus mirabilis, Morganella morganii, Providencia rettgeri, Proteus vulgaris, Pseudomonas, Enterobacter, and Enterococci.). Also indicated in the treatment of prostatitis (due to susceptible strains Escherichia coli Enterococcus (S. faecalis), Proteus mirabilis Enterobacter sp.).
Definition
ChEBI: Carindacillin is a penicillin. It is a conjugate acid of a carindacillin(1-).
Manufacturing Process
(A) Preparation of Phenylchlorocarbonyl Ketene: To phenylmalonic acid (20 g)
in ethyl ether (100 ml) there is added phosphorus pentachloride (46 g). A
vigorous reaction occurs. The reaction mixture is refluxed for 4 hours then the
ether partially removed by heating on a steam bath. The reaction mixture
becomes black when about half the ether is removed and the remaining ether
is removed under reduced pressure (at 100 mm). The residue is distilled
under vacuum and the fraction boiling at 75° to 90°C at 1.5 to 4 mm
collected. The product, a yellow liquid, is redistilled at 74°C and 1.5 mm. It
shows a strong peak in the infrared region of the spectrum at 4.69 mu.
Repetition of this procedure but using 10 g of phenylmalonic acid instead of
20 g produces a less vigorous reaction on addition of the phosphorus
pentachloride. The same product is obtained.
(B) Acylation of 6-Aminopenicillanic Acid: To a solution of the aryl
halocarbonyl ketene (0.1 mol) in methylene chloride (sufficient to provide a
clear solution and generally from about 5 to 10 ml per gram of ketene) there
is added the proper alcohol R2OH (0.1 mol), in this case 5-indanyl alcohol.
The reaction mixture is maintained under an atmosphere of nitrogen and
stirred for a period of from 20 minutes to 3 hours, care being taken to exclude moisture. The temperature may range from about -70° to about -
20°C. The infrared spectrum of the mixture is then taken to determine and
confirm the presence of the ketene ester. A solution of 6-aminopenicillanic
acid-triethylamine salt (0.1 mol) in methylene chloride (50 ml) is added and
the mixture stirred at -70° to -20°C for 10 minutes. The cooling bath is then
removed and the reaction mixture stirred continuously and allowed to warm to
room temperature.
Various isolation methods are then spelled out in US Patent 3,679,801.
brand name
Geocillin (Pfizer).
Therapeutic Function
Antibacterial
Metabolism
Not Available
Safety information for CARINDACILLIN
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
