Calcium oxide
Synonym(s):Calcium oxide from marble;Lime;Lime, caustic, Quicklime;Quicklime
- CAS NO.:1305-78-8
- Empirical Formula: CaO
- Molecular Weight: 56.08
- MDL number: MFCD00010911
- EINECS: 215-138-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:34
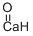
What is Calcium oxide?
Description
Calcium oxide (CaO, CAS Reg. No. 1305-78-8) is also known as lime, quick lime, burnt lime, or calx. Lime does not occur naturally since it reacts so readily with water (to form hydrated lime) and carbon dioxide (to form limestone). It is produced from calcium carbonate, limestone, or oyster shells by calcination at temperatures of 1,700-2,450℃.
Calcium Oxide is a solid with a very high affinity for water - it will react with water in the air, or in your skin or anywhere it can and form calcium hydroxide. This reaction is exothermic so it releases a lot of heat while it is reacting - there fore as well as being corrosive and causing significant skin irritation, calcium oxide's reaction with water can also cause burns. Calcium hydroxide is basically hydrated calcium oxide. It is alkali so can be corrosive. In solution it makes limewater.
CaO is not found pure in nature but rather is contained in various abundant minerals (i.e. calcite, aragonite, limestone, marble) but vary greatly in their purity (impurities usually include magnesia, iron, alumina, silica, sulfur). Of these iron and sulfur are most troublesome (i.e. where clarity is important in glass). Lime minerals vary in the degree of crystallization and cohesion of the crystalline mass and the homogeneity of the matrix.
Calcium oxide is the principle flux in medium and high temperature glazes, beginning its action (within the glaze) around 1100C. It must be used with care in high-fire bodies because its active fluxing action can produce a body that is too volatile (melting if slightly overfired).
Lime, or calcium oxide, is a principle ingredient in the production of Portland cement, the basis for most mortars and concrete. Hydrated or ‘slaked’ lime is the chemical calcium hydroxide. This chemical is also used in mortars. Both types of lime are strong bases and are also used in food production (calcium hydroxide is commonly used in making corn tortillas), petroleum refining and sewage treatment. In the household it is used by aquarium hobbyists to add bioavailable calcium to fish tanks. It is also found in hair relaxers.
Chemical properties
Calcium oxide, CaO, occurs as white or grayish-white lumps or granular powder. The presence of iron gives it a yellowish or brownish tint.
Physical properties
Calcium oxide is a white caustic crystalline alkali substance that goes by the common name lime. The term lime is used both generically for several calcium compounds and with adjectives to qualify different forms of lime. This entry equates lime, also called quicklime or burnt lime, with the compound calcium oxide. Hydrated lime, made by combining lime with water, is calcium hydroxide and is often referred to as slaked lime (Ca(OH)2). Dolomite limes contain magnesium as well as calcium. Limestone is the compound calcium carbonate. The term lime comes from the Old English word l?m for a sticky substance and denotes lime’s traditional use to produce mortar. Calx was the Latin word for lime and was used to name the element calcium.
History
Calcium oxide dates from prehistoric times. It is produced by heating limestone to drive off carbon dioxide in a process called calcination: CaCO3(s) CaO(s) + CO2(g). At temperatures of several hundred degrees Celsius, the reaction is reversible and calcium oxide will react with atmospheric carbon dioxide to produce calcium carbonate. Efficient calcium oxide production is favored at temperatures in excess of 1,000°C. In prehistoric times limestone was heated in open fires to produce lime. Over time, lined pits and kilns were used to produce lime. Brick lime kilns were extensively built starting in the 17th century and the technology to produce lime has remained relatively constant since then.
The Uses of Calcium oxide
Calcium Oxide is a general food additive consisting of white granules or powder of poor water solubility. it is obtained by heating limestone (calcium carbonate) in a furnace. it is also termed lime or quicklime. it is used as an anticaking agent, firming agent, and nutritive supple- ment in applications such as grain products and soft candy.
The Uses of Calcium oxide
In bricks, plaster, mortar, stucco and other building and construction materials; manufacture of steel, aluminum, magnesium, and flotation of non-ferrous ores; manufacture of glass, paper, Na2CO3 (Solvay process), Ca salts and many other industrial chemicals; dehairing hides; clarification of cane and beet sugar juices; in fungicides, insecticides, drilling fluids, lubricants; water and sewage treatment; in laboratory to absorb CO2 (the combination with NaOH is known as soda-lime, q.v.).
The Uses of Calcium oxide
The major uses of lime are metallurgy, flue gas desulfurization, construction, mining, papermaking, and water treatment. About one third of calcium oxide production in the United States is used for metallurgical processes, principally in the iron and steel industry. Calcium oxide is used to remove impurities during the refining of iron ore. Calcium oxide combines with compounds such as silicates, phosphates, and sulfates contained in iron ores to form slag. Lime is also used for purification in other metal refining and to control pH in mining processes such as leaching and precipitation. The calcium oxide is also used in remediation of mine wastes to recover cyanides and to neutralize acid mine drainage.
What are the applications of Application
Calcium oxide is known as quicklime or burnt lime, is a widely used chemical compound
Definition
ChEBI: Calcium oxide is a member of the class of calcium oxides of calcium and oxygen in a 1:1 ratio. It has a role as a fertilizer.
Production Methods
Calcium oxide is commercially obtained from limestone. The carbonate is roasted in a shaft or rotary kiln at temperatures below 1,200°C until all CO2 is driven off. The compound is obtained as either technical, refractory or agri cultural grade product. The commercial product usually contains 90 to 95% free CaO. The impurities are mostly calcium carbonate, magnesium carbon ate, magnesium oxide, iron oxide and aluminum oxide.
Aroma threshold values
Aroma at 1.0%: intense, high impacting fresh sweet juicy lime, citral with a distilled lime note, cool and refreshing with green juicy nuances.
Taste threshold values
Taste characteristics at 10 ppm in 5% sugar and 0.1% CA; intense fresh tangy lime juice, citrus citral candy lime character with notes of West Indian lime
General Description
Calcium oxide appears as an odorless, white or gray-white solid in the form of hard lumps. A strong irritant to skin, eyes and mucous membranes. Used in insecticides and fertilizers.
Air & Water Reactions
Crumbles on exposure to moist air. Reacts with water to form corrosive calcium hydroxide, with evolution of much heat. Temperatures as high as 800° C have been reached with addition of water (moisture in air or soil). The heat of this reaction has caused ignition of neighboring quantities of sulfur, gunpowder, wood, and straw [Mellor 3: 673 1946-47].
Reactivity Profile
A base and an oxidizing agent. Neutralizes acids with generation of heat. Nonflammable, but will support combustion by liberation of oxygen, especially in the presence of organic materials. Reacts very violently with liquid hydrofluoric acid [Mellor 2, Supp. 1:129 1956]. Reacts extremely violently with phosphorus pentaoxide when reaction is initiated by local heating [Mellor 8 Supp.3:406 1971].
Hazard
Evolves heat on exposure to water. Danger- ous near organic materials. Upper respiratory tract irritant.
Health Hazard
Causes burns on mucous membrane and skin. Inhalation of dust causes sneezing.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Vapors may accumulate in confined areas (basement, tanks, hopper/tank cars etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Flammability and Explosibility
Not classified
Agricultural Uses
Calcium oxide (CaO) is a white powder with a neutralizing value or calcium carbonate equivalent (CCE) of 179%, compared to 100% for calcium carbonate (CaCO3). For quick results, either calcium oxide or calcium hydroxide [Ca(OH)2] is used. Calcium oxide is also known as lime, unslaked lime, burned lime or quicklime. Roasting CaCO3 in a furnace makes calcium oxide. A complete mixing of calcium oxide with soil is difficult because it cakes due to absorption of water.
Industrial uses
Lime is the most widely used reagent in the mineral industry for flotation of sulfides and, in some cases, non-sulfide minerals. The word “lime” is a general term used to describe any kind of calcareous material or finely divided form of limestone and dolomite. In more strict chemical terms, lime is calcinated limestone known as calcium oxide (CaO), quicklime or unslaked lime.The slaked or hydrated lime Ca(OH)2 is the form of lime primarily used in mineral flotation. Production of high-calcium lime is based on calcination of limestone at a temperature of 1100–1300 °C in kilns.
CaCO3+heat--->CaO+CO2 For high-magnesium (dolomitic) limestone, the calcination reaction (at 1000–1200 °C) is CaCO3·MgCO3 (limestone) + heat--->CaOMgO (quicklime-2CO2)
Safety Profile
A caustic and irritating material. See also CALCIUM COMPOUNDS. A common air contaminant. A powerful caustic to living tissue. The powdered oxide may react explosively with water. Mixtures with ethanol may igmte if heated and thus can cause an air-vapor explosion. Violent reaction with (I3203 + CaCl2) interhalogens (e.g., BF3, CIF3), F2, HF, P2O5 + heat, water. Incandescent reaction with liquid HF. Incompatible with phosphoms(V) oxide.
Potential Exposure
Calcium oxide is used as a refractory material; a binding agent in bricks; plaster, mortar, stucco, and other building materials. A dehydrating agent, a flux in steel manufacturing, and a labora
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin,remove contaminated clothing and wash immediately withsoap and water. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart actionhas stopped. Transfer promptly to a medical facility. Whenthis chemical has been swallowed, get medical attention. Ifvictim is conscious, administer water or milk. Do not inducevomiting. Medical observation is recommended for 24-48 hafter breathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consider administering a corticosteroidspray.
Storage
Color Code—Green: General storage may be used.Prior to working with calcium oxide you should be trainedon its proper handling and storage. Should be stored on dryflooring in a fire-resistant room, well protected from theweather. The area should be cool and adequately ventilated.Store in containers protected from physical damage, acidsand oxidizing materials, such as permanganate, dichromate,or chlorine.
Shipping
UN1910 Calcium oxide, Hazard class: 8; Labels: 8-Corrosive material.
Incompatibilities
The water solution is a medium strong base. Reacts with water, forming calcium hydroxide and sufficient heat to ignite nearby combustible materials. Reacts violently with acids, halogens, metals.
Waste Disposal
Pretreatment involves neutralization with hydrochloric acid to yield calcium chloride. The calcium chloride formed is treated with soda ash to yield the insoluble calcium carbonate. The remaining brine solution may be discharged into sewers and waterways
Properties of Calcium oxide
| Melting point: | 2570 °C |
| Boiling point: | 2850 °C (lit.) |
| Density | 3.3 g/mL at 25 °C (lit.) |
| refractive index | 1.83 |
| Flash point: | 2850°C |
| storage temp. | no restrictions. |
| solubility | 1.65g/l Risk of violent reaction. |
| form | powder |
| color | White to yellow-very slightly beige |
| Specific Gravity | 3.3 |
| Odor | wh. or gray cryst. or powd., odorless |
| PH | 12.6 (H2O, 20℃)(saturated solution) |
| Water Solubility | REACTS |
| Sensitive | Air & Moisture Sensitive |
| Crystal Structure | Cubic |
| Merck | 14,1686 |
| Dielectric constant | 2.2(Ambient) |
| Exposure limits | ACGIH: TWA 2 mg/m3 OSHA: TWA 5 mg/m3 NIOSH: IDLH 25 mg/m3; TWA 2 mg/m3 |
| Stability: | Stability Stable, but absorbs carbon dioxide from the air. Incompatible with water, moisture, fluorine, strong acids. |
| CAS DataBase Reference | 1305-78-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Calcium monoxide(1305-78-8) |
| EPA Substance Registry System | Calcium oxide (1305-78-8) |
Safety information for Calcium oxide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Calcium oxide
| InChIKey | ODINCKMPIJJUCX-UHFFFAOYSA-N |
Calcium oxide manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 CALCIUM OXIDE POWDER 99%View Details
CALCIUM OXIDE POWDER 99%View Details -
 Calcium Oxide 99%View Details
Calcium Oxide 99%View Details -
 Calcium oxide 99%View Details
Calcium oxide 99%View Details -
 Calcium oxide CAS 1305-78-8View Details
Calcium oxide CAS 1305-78-8View Details
1305-78-8 -
 Calcium oxide CAS 1305-78-8View Details
Calcium oxide CAS 1305-78-8View Details
1305-78-8 -
 Calcium oxide CAS 1305-78-8View Details
Calcium oxide CAS 1305-78-8View Details
1305-78-8 -
 Calcium oxide CAS 1305-78-8View Details
Calcium oxide CAS 1305-78-8View Details
1305-78-8 -
 Calcium Oxide Nano PowderView Details
Calcium Oxide Nano PowderView Details
12031-95-7
