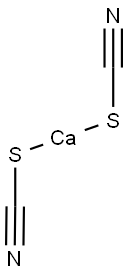
CALCIUM CYANIDE
- CAS NO.:592-01-8
- Empirical Formula: C2CaN2
- Molecular Weight: 92.11
- MDL number: MFCD00049398
- EINECS: 209-740-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-14 15:18:27
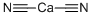
What is CALCIUM CYANIDE?
Description
Calcium cyanide is used mainly for the extraction or cyanidation of gold and silver ores. It is also used in the production of prussiates or ferrocyanides, in the froth flotation of minerals, in processes where gold complexes are adsorbed on carbon, in the manufacture of stainless steel, as a fumigant and rodenticide, and as a cement stabiliser. The main users of cyanides are the steel, electroplating, mining, and chemical industries. The principal cyanide compounds used in industrial operations are potassium and sodium cyanide and calcium cyanide, particularly in metal leaching operations. Cyanides have been well established in uses as insecticides and fumigants; in the extraction of gold and silver ores; in metal cleaning; in the manufacture of synthetic fibres, various plastics, dyes, pigments, and nylon; and as reagents in analytical chemistry. Calcium cyanide decomposes on heating above 350°C, producing toxic fumes including nitrogen oxides and HCN. It reacts violently with water, moist air, carbon dioxide, acids, and acid salts producing highly toxic and flammable HCN. It reacts violently when heated with oxidising substances causing fire and explosion hazard.
Chemical properties
Calcium cyanide is a white crystalline solid or powder. Odor of hydrogen cyanide.
The Uses of CALCIUM CYANIDE
Calcium cyanide is used for the extraction of gold and silver from their ores, in the froth flotation of minerals, as a fumigant, and as a rodenticide.
The Uses of CALCIUM CYANIDE
Fumigant; rodenticide; in stainless-steel manufacture; in leaching ores of precious metals; stabilizer for cement.
Production Methods
Calcium cyanide is made commercially from lime, calcium oxide, coke, and nitrogen. The reactions are carried out in an electric furnace. The resulting melt is cooled rapidly to prevent reversion to calcium cyanamide. The product is marketed in the form of flakes, which are dark gray because of the presence of carbon. The extraction or cyanidation of precious-metal ores was the first and is still the largest use for calcium cyanide.
General Description
White crystals or powder or gray-black powder (technical grade). Toxic by skin absorption through open wounds, by ingestion, and by inhalation.
Air & Water Reactions
Water soluble with evolution of some hydrogen cyanide, a flammable poison gas. Release of gas is much more rapid if acid is present.
Reactivity Profile
CALCIUM CYANIDE gives weakly acidic solutions. Contact with acids causes rapid evolution of hydrogen cyanide. Incompatible with isocyanates, nitrides, and peroxides. May react rapidly with oxidizing agents.
Hazard
Toxic by ingestion and skin absorption.
Health Hazard
Inhalation or ingestion causes headache, nausea, vomiting and weakness; high concentrations are rapidly fatal.
Health Hazard
Calcium cyanide is a highly poisonous compound to humans, animals, and fish. The toxic routes are ingestion, skin contact, and inhalation of the dust. It forms HCN readily when it reacts with CO2 or water. This makes it highly hazardous, more so than the alkalimetal cyanides, although the LD50 value of Ca(CN)2 is greater than the sodium or potassium cyanides.
LD50 value, oral (rats): 39 mg/kg.
Fire Hazard
Special Hazards of Combustion Products: Decomposes in fire to give very toxic gases, including hydrogen cyanide.
Safety Profile
A deadly poison by ingestion and probably other routes. When heated to decomposition it emits toxic fumes of NO, and CN-. See also CALCIUM COMPOUNDS and CYANIDE
Potential Exposure
Calcium cyanide is used as a fumigant; as a rodenticide; in leaching precious metal ores; in the manufacture of stainless steel; and as a stabilizer forcement. Used as raw material for production of nitrogenous compounds and in treatment of alcoholism
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Do notallow water to enter nose or mouth. Seek medical attentionimmediately. If this chemical contacts the skin, removecontaminated clothing and wash immediately with soap andwater. If this chemical has been inhaled, remove from expo?sure, begin rescue breathing (using universal precautions,including resuscitation mask) if breathing has stopped andCPR if heart action has stopped. Transfer promptly to a532 Calcium cyanidemedical facility. When this chemical has been swallowed,get medical attention. Give large quantities of water andinduce vomiting. Do not make an unconscious personvomit. Medical observation is recommended for 24-48 hafter breathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray. Use amyl nitrate capsules if symptoms of cyanide poisoning develop. All area employees should betrained regularly in emergency measures for cyanidepoisoning and in CPR. A cyanide antidote kit should bekept in the immediate work area and must be rapidly available. Kit ingredients should be replaced every 1-2 years toensure freshness. Persons trained in the use of this kit,oxygen use, and CPR must be quickly available.
storage
Calcium cyanide is stored in tight containers free from moisture. Proper ventilation and protective equipment should be used while handling the solid or while preparing an aqueous solution. It is shipped in mild-steel or fiber drums.
Shipping
UN1575 Calcium cyanide, Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Incompatibilities
Contact with water, acids, acidic salts; moist air, or carbon dioxide, forms highly toxic and flammable hydrogen cyanide. Incompatible with fluorine, magnesium. Reacts violently when heated with nitrites, nitrates, chlorates, and perchlorates. Calcium cyanide decomposes in high heat forming hydrogen cyanide and nitrous oxides fumes
Waste Disposal
Add cyanide waste to strong alkaline sodium hypochlorite. Let stand 24 hours then flush to sewage plant.
Properties of CALCIUM CYANIDE
| Melting point: | 640 estimated [KIR78] |
| Density | 1.8 g/cm3 |
| solubility | soluble in H2O, ethanol |
| form | white rhombohedral crystals |
| color | white rhomb crystals, crystalline; hygroscopic |
| Water Solubility | soluble H2O, gradually releasing HCN [MER06] |
| Exposure limits | TLV-TWA (measured as CN) skin 5 mg(CN)/ m3 (ACGIH); 5 mg(CN)/m3/10-min ceiling (NIOSH). |
| EPA Substance Registry System | Calcium cyanide (592-01-8) |
Safety information for CALCIUM CYANIDE
Computed Descriptors for CALCIUM CYANIDE
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Mefenamic Acid IP/BP/EP/USP Diclofenac Sodium IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4