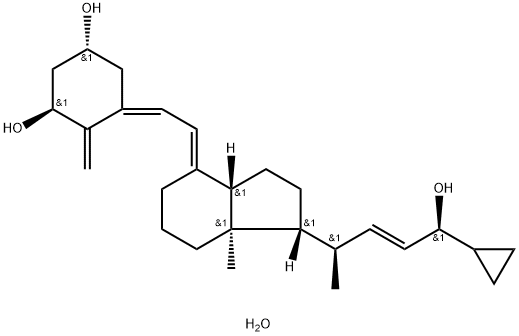
Calcipotriene
Synonym(s):(5Z,7E,22E,24S)-24-Cyclopropyl-9,10-secochola-5,7,10(19),22-tetraene-1alpha,3beta,24-triol;Calcipotriene
- CAS NO.:112965-21-6
- Empirical Formula: C27H40O3
- Molecular Weight: 412.6
- MDL number: MFCD00866630
- EINECS: 601-218-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-15 14:23:52
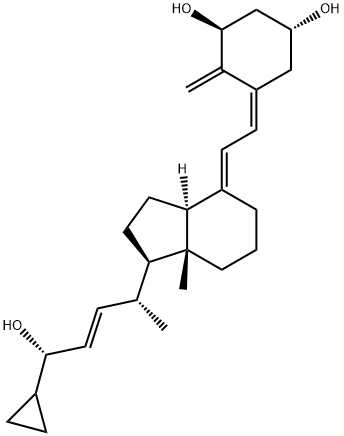
What is Calcipotriene?
Absorption
Clinical studies with radiolabeled ointment indicate that approximately 6% (+3%, SD) of the applied dose of calcipotriene is absorbed systemically when the ointment is applied topically to psoriasis plaques or 5% (+2.6%, SO) when applied to normal skin.
Toxicity
Topically applied calcipotriene can be absorbed in sufficient amounts to produce systemic effects. Elevated serum calcium has been observed with excessive use of calcipotriene.
Description
Calcipotriene also known as Calcipotriol, is a topical vitaminD3 derivative effective in the treatment of psoriasis vulgaris. The drug acts by binding to vitamin D3 receptors in the skin keratinocytes, producing an elevation in cell differentiation and a reduction in cell proliferation. Although its efficacy is comparable to calcitriol, calcipotriol exhibits at least 100 times less effect on calcium metabolism in rats.
Chemical properties
White Crystalline Solid
Originator
Leo Denmark (Denmark)
The Uses of Calcipotriene
Calcipotriene is an antipsoriatic that is a vitamin D3 analogue with low calcemic activity. It works by regulating the production and growth of skin cells. In the United States, this drug was first marketed under the trade name Dovonex and is used mainly for the treatment of psoriasis. It is one of the most effective non glucocorticoid topical drugs for the treatment of localized psoriasis.This drug has many off-label applications that are alternative therapies for many dermatologic diseases.
Indications
For the treatment of moderate plaque psoriasis in adults.
Background
Calcipotriol (INN) or calcipotriene (USAN) is a sythetic derivative of calcitriol or Vitamin D.
What are the applications of Application
Calcipotriol is vitamin D3 analog
Indications
Calcipotriene (Dovonex), a synthetic vitamin D3 derivative, is indicated for the treatment of moderate plaque psoriasis. Its mechanism of action is unknown, although it competes for calcitriol receptors on keratinocytes and normalizes differentiation. It also has a variety of immunomodulatory effects in the skin. Although the drug can cause local irritation, the most serious toxicities are hypercalciuria and hypercalcemia, which are usually reversible.
Definition
ChEBI: Calcipotriol is a seco-cholestane that is 26,27-cyclo-9,10-secocholesta-5,7,10,22-tetraene carrying additional hydroxy substituents at positions 1, 3 and 24. It is used (as its hydrate) in combination with betamethasone dipropionate, a corticosteroid, for the topical treatment of plaque psoriasis in adult patients. It has a role as a drug allergen and an antipsoriatic. It is a member of cyclopropanes, a secondary alcohol, a triol, a hydroxy seco-steroid and a seco-cholestane.
Preparation
A convergent approach for the total synthesis of calcipotriol (brand name: Dovonex), a proven Vitamin D analog used for the treatment of psoriasis, and medicinally relevant synthetic analogs is described.
A Synthesis of calcipotriol via a Key Electroreductive Cross-coupling Approach
Calcipotriol is currently the most successful VitD analog and is prescribed for the treatment of psoriasis, an autoimmune skin disease.
Manufacturing Process
(1S,3R)-Bis-(t-butyldimethylsilyloxy)-(20S)-formyl-9,10-secopregna(5E,7E,10
(19))triene (Calverley Tetrahedron 43.4609 (1967) and (cyclopropyl)(tri-phenylphoshoranylidene)ketone are stirred in dimethyl sulfoxide under
nitrogen. The reaction mixture is then diluted at room temperature with ethyl
acetate and washed with common salt solution. The organic phase is dried on
sodium sulfate and filtered. After removal of the solvent, the residue is filtered
with toluene through silica gel. Evaporation of the solvent and gradient
chromatography (toluene/hexane (1:1)-toluene) of the residue on silica gel
yield (5E,7E,22E),(1S,3R)-1,3-bis-(t-butyldimethylsilyloxy)-24-cyclopropyl-
9,10-secochola-5,7,10(19),22-tetraene-24-one.
(5E,7E,22E),(1S,3R)-1,3-Bis-(t-butyldimethylsilyloxy)-24-cyclopropyl-9,10-
secochola-5,7,10(19),22-tetraene-24-one in tetrahydrofuran and methanol are
mixed with a 0.4 M methanol CeCl3·7H2O solution. Sodium borohydride is
added by portions under nitrogen with ice cooling. The suspension is stirred
with ice cooling and then put into ice/common salt solution. The aqueous
phase is extracted with ethyl acetate, the organic phase is washed neutral
with water and dried on sodium sulfate. Filtration and removal of the solvent
yield oil. By chromatography on silica gel with ethyl acetate/hexane (1:9). The
(5E,7E,22E),(1S,3R,24S)-1,3-bis-(t-butyldimethylsilyloxy)-24-cyclopropyl-
9,10-secochola-5,7,10(19),22-tetraene-24-ol is obtained.
(5E,7E,22E),(1S,3R,24S)-1,3-Bis-(t-butyldimethylsilyloxy)-24-cyclopropyl-
9,10-secochola-5,7,10(19),22-tetraene-24-ol is dissolved in toluene and after
addition of anthracene and 1 drop of triethylamine it is radiated at room
temperature with a high pressure mercury vapor lamp (Heraeus TQ 150)
through Pyrex glass. The reaction mixture is concentrated by evaporation and
the residue a mixture of (5Z,7E,22E),(1S,3R,24S)-1,3-bis-(t-butyldimethylsilyloxy)-24-cyclopropyl-9,10-secochola-5,7,10(19),22-tetraene-
24-ol and anthracene - is directly reacted with tetrabutylammonium fluoride.
(5Z,7E,22E),(1S,3R,24S)-1,3-Bis-(t-butyldimethylsilyloxy)-24-cyclopropyl-
9,10-secochola-5,7,10(19),22-tetraene-24-ol in tetrahydrofuran is kept with a
1 M solution of tetrabutylammonium fluoride in tetrahydrofuran under
nitrogen. For working up, the cooled reaction mixture is poured into cold
sodium bicarbonate solution and then extracted with ethyl acetate. After
drying of the organic phase on sodium sulfate, filtration and evaporation of
the solvent yields a resin-like residue. Chromatography on silica gel with ethyl
acetate/hexane (2:1) yields (5Z,7E,22E),(1S,3R,24S)-24-cyclopropyl-9,10-
secochola-5,7,10(19),22-tetraene-1,3,24-triol.
brand name
Dovonex (Leo);Daivonex.
Therapeutic Function
Antipsoriatic
Biological Activity
Vitamin D 3 analog that displays minimal effects on calcium homeostasis. Regulates cell differentiation and proliferation; exhibits antiproliferative activity against human HL-60, HL60/MX2, MCF-7, T47D, SCC-25 and mouse WEHI-3 cancer cell lines.
Biochem/physiol Actions
Calcipotriol, a synthetic derivative of calcitriol or Vitamin D, is used in the treatment of psoriasis and marketed under the trade name Dovonex. It has comparable affinity with calcitriol (Vit. D) for the Vitamin D receptor (VDR), while being less than 1% as active as the calcitriol in regulating calcium metabolism. VDR belongs to the steroid/thyroid receptor superfamily, and is found on the cells of many different tissues including the thyroid, bone, kindney, and T cells of the immune system. Binding of calcipotriol to the VDR modulates the T cells gene transcription of cell differentiation and proliferation-related genes.
Pharmacokinetics
Calcipotriene is a synthetic analog of vitamin D. In humans, the natural supply of vitamin D depends mainly on exposure to the ultraviolet rays of the sun for conversion of 7-dehydrocholesterol to vitamin D3 (cholecalciferol) in the skin.
Pharmacology
Calcipotriene (Dovonex) enhances the effectiveness of ultraviolet B (UVB) but also increases photosensitivity in UVB-treated patients. Ultraviolet light, 6% salicylic acid, 12% lactic acid, hydrocortisone valerate 0.2% ointment, and tazarotene (Tazorac) gel degrade calcipotriene (Dovonex). Halobetasol ointment and 5% tar gel are compatible with Calcipotriene (Dovonex). A British study found calcipotriene (Dovonex) to be safe and effective in a pediatric population over the age of 3, although it is not approved by the FDA.
Side Effects
Possible side effects include:
severe burning, stinging, skin rash, or other irritation after applying the medicine;
worsening of your skin condition;
high calcium levels--confusion, tiredness, nausea, vomiting, loss of appetite, constipation, increased thirst or urination, weight loss;
mild skin irritation;
skin rash;
itching.
Metabolism
Hepatic. Calcipotriene metabolism following systemic uptake is rapid, and occurs via a similar pathway to the natural hormone. The primary metabolites are much less potent than the parent compound.
Storage
Store at -20°C
Properties of Calcipotriene
| Melting point: | 166-168°C |
| Boiling point: | 582.0±50.0 °C(Predicted) |
| Density | 1.12±0.1 g/cm3(Predicted) |
| storage temp. | Desiccate at -20°C |
| solubility | DMSO: soluble15mg/mL, clear |
| form | powder |
| pka | 14.29±0.20(Predicted) |
| color | White to off-white |
| Stability: | Light and Temperature Sensitive, Light And Temperature Sensitive |
| CAS DataBase Reference | 112965-21-6(CAS DataBase Reference) |
Safety information for Calcipotriene
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H300:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Calcipotriene
| InChIKey | LWQQLNNNIPYSNX-HCHVWAPNSA-N |
| SMILES | [C@@H]1(O)C/C(=C/C=C2\CCC[C@@]3(C)[C@@]\2([H])CC[C@@H]3[C@H](C)/C=C/[C@H](C2CC2)O)/C(=C)[C@@H](O)C1 |
Calcipotriene manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
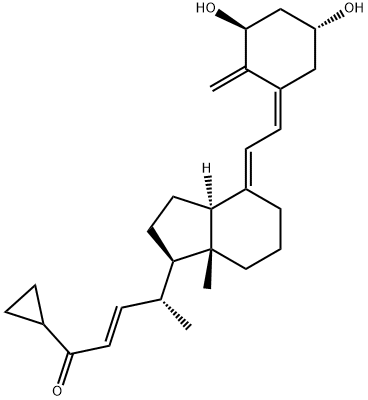
![(2E,4R)-4-[(1R,3aS,4E,7aR)-4-[(2E)-2-[(3S,5R)-3,5-Bis[[(tert-butyl)dimethylsilyl]oxy]-2-methylenecyclohexylidene]ethylidene]octahydro-7a-methyl-1H-inden-1-yl]-1-cyclopropyl-2-penten-1-one](https://img.chemicalbook.in/CAS/GIF/112849-17-9.gif)

You may like
-
 112965-21-6 Calcipotriol 99%View Details
112965-21-6 Calcipotriol 99%View Details
112965-21-6 -
 112965-21-6 99%View Details
112965-21-6 99%View Details
112965-21-6 -
 Calcipotriol 98%View Details
Calcipotriol 98%View Details
112965-21-6 -
 Calcipotriol 112965-21-6 98%View Details
Calcipotriol 112965-21-6 98%View Details
112965-21-6 -
 112965-21-6 Calcipotriol 99%View Details
112965-21-6 Calcipotriol 99%View Details
112965-21-6 -
 Calcipotriene CAS 112965-21-6View Details
Calcipotriene CAS 112965-21-6View Details
112965-21-6 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
