Butyraldehyde oxime
- CAS NO.:110-69-0
- Empirical Formula: C4H9NO
- Molecular Weight: 87.12
- MDL number: MFCD00013943
- EINECS: 203-792-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32

What is Butyraldehyde oxime?
Chemical properties
colourless liquid
The Uses of Butyraldehyde oxime
Butyraldoxime can be used to create an anti-counterfeiting ink formulation.
Definition
ChEBI: An aldoxime derived from n-butanal.
Preparation
To a slowly stirred mixture of 73 gm (1 mole) of butylamine and 109 gm of a 12.2% aqueous solution of sodium tungstate is added (1.75 hr) with cooling to maintain a temperature of 15°C, 220 gm of 28% hydrogen peroxide. During this reaction 130 ml of ethanol is added portionwise to clarify the emulsion which tends to form. The green solution is stirred at 15°C for an additional hour after the addition has been completed. The reaction mixture is cooled, neutralized, and freed of the ethanol under reduced pressure. The solution is then saturated with sodium chloride and the oily product is separated and distilled to afford 50 gm (58%), b.p. 152°C.
Evidently hydroxylamines may also be oxidized with hydrogen peroxide in the presence of sodium tungstate, as mentioned above. In a recent report, phenylglyoxime was prepared by a ferric chloride oxidation of the correspondingly hydroxylamine oxime.
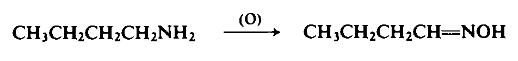
General Description
A liquid. Flash point 140°F. Slightly less dense than water. Vapors heavier than air. Used to make plastics and rubber.
Air & Water Reactions
Flammable. Slightly soluble in water.
Reactivity Profile
Butyraldehyde oxime is highly explosive during vacuum distillation. Butyraldehyde oxime is incompatible with oxidizing materials. Butyraldehyde oxime is also incompatible with metallic impurities. Butyraldehyde oxime may react with strong acids.
Health Hazard
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
A poison by intraperitoneal route. Mutation datareported. Flammable liquid when exposed to heat or flame. To fight fire, use alcohol foam, dry chemical. Highly explosive. Can explode during vacuum disdlation. Incompatible with oxidzing materials, metallic impurities. When heated to decomposition it emits toxic fumes of NOx. See also ALDEHYDES.
Properties of Butyraldehyde oxime
| Melting point: | -29.5°C |
| Boiling point: | 152-154 °C |
| Density | 0.923 |
| refractive index | 1.4367 |
| Flash point: | 58 °C |
| storage temp. | Refrigerator, under inert atmosphere |
| solubility | Chloroform, Methanol (Slightly) |
| form | Oil |
| pka | 11.03±0.11(Predicted) |
| color | Colourless |
| Water Solubility | <0.1 g/100 mL at 20 ºC |
| BRN | 1698799 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, powdered metals, acids. |
| CAS DataBase Reference | 110-69-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Butanal, oxime(110-69-0) |
| EPA Substance Registry System | Butanal oxime (110-69-0) |
Safety information for Butyraldehyde oxime
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H226:Flammable liquids H302:Acute toxicity,oral H311:Acute toxicity,dermal H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P370+P378:In case of fire: Use … for extinction. P405:Store locked up. P403+P235:Store in a well-ventilated place. Keep cool. P501:Dispose of contents/container to..… |
Computed Descriptors for Butyraldehyde oxime
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Butyraldoxime CAS 110-69-0View Details
Butyraldoxime CAS 110-69-0View Details
110-69-0 -
 BUTYRALDOXIME CAS 110-69-0View Details
BUTYRALDOXIME CAS 110-69-0View Details
110-69-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1