5,5-DIMETHYL-1-PYRROLINE N-OXIDE
Synonym(s):DMPO
- CAS NO.:3317-61-1
- Empirical Formula: C6H11NO
- Molecular Weight: 113.16
- MDL number: MFCD00005279
- EINECS: 222-011-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-15 21:27:52
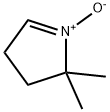
What is 5,5-DIMETHYL-1-PYRROLINE N-OXIDE?
Description
Free radicals are highly reactive, short-
Chemical properties
white to faintly yellow solid
The Uses of 5,5-DIMETHYL-1-PYRROLINE N-OXIDE
5,5-Dimethyl-1-pyrroline N-oxide is a reagent generally used either as a free-radical spin-trapping agent, or electrophilic component during the synthesis of pyrrolidine derivatives. It may also be considered as 1,3-dipole in cycloaddition processes.
The Uses of 5,5-DIMETHYL-1-PYRROLINE N-OXIDE
Spin trap agent for biological systems.
The Uses of 5,5-DIMETHYL-1-PYRROLINE N-OXIDE
5,5-Dimethyl-1-pyrroline N-oxide has been used as a spin trapping reagent to detect free radicals in electron paramagnetic resonance (EPR) based reactions.
What are the applications of Application
DMPO is a cell permeable hydrophillic spin trap agent for superoxide detection
Preparation
To a three-necked round bottom flask equipped with a condenser, addition funnel, thermometer, and mechanical stirrer and containing 300 ml. 95% ethanol precooled to 2°C is added, all at once, 14.5 g (0.1 mole) 4-methyl-4-nitropentanol, and 13.1 g (1.2 mole) zinc dust. With rapid agitation, the glacial acetic acid (24.0 g, 0.4 mole) is added dropwise over a 1-hr period while maintaining the reaction temperature below 15°C. The mixture is stirred vigorously for 2 hr and then stored in the refrigerator for 2 days, at approximately 1°C. Then the zinc acetate is filtered and rinsed with 100 ml of ethanol. The combined ethanol factions are rotoevapo-rated to give the crude nitrone. The crude nitrone is dissolved in a 200 ml portion of dichloromethane and the latter washed two times with saturated sodium bicarbonate solution. The organic layer is dried over sodium sulfate and the solvent rotoevaporated, to give 10.7 (94% yield) of the crude nitrone. The product was purified by double distillation (b.p. 53°C, 0.1 Torr), to give 6.8 g (60%) of the pure nitrone as a white hygroscopic solid. The H1 NMR (400 MH CDC13, Me4Si) is 6.80 (t, 2H, methylene bound to quaternary C, J = 7.2Hg), 1.43 (s, 6H, methyl), C13NMr (100 MH2, CDC13, Me4 Si) 5=132.4 (Vinyl), 73.5 (quaternary), 34.1 (Allyl), 25.3 (methylene bound to quaternary C), 24.4 (methyls).

Definition
ChEBI: 5,5-dimethyl-1-pyrroline N-oxide is a member of the class of 1-pyrroline nitrones (1-pyrroline N-oxides) resulting from the formal N-oxidation of 5,5-dimethyl-1-pyrroline. Used as a spin trap for the study of radicals formed by enzymatic acetaldehyde oxidation. It has a role as a neuroprotective agent and a spin trapping reagent. It is functionally related to a 5,5-dimethyl-1-pyrroline.
Biological Activity
Water soluble nitric oxide spin trap; allows the measurement of oxygen-centered free radicals in biological systems at room temperature using electron spin resonance (ESR). Has a high reaction rate constant for superoxide and hydroxyl radicals, and distinguishes simultaneously among a variety of important biologically generated free radicals.
Physiological effects
5,5-dimethyl-1-pyrroline N-oxide is used as a spin trap for the study of radicals formed by enzymatic acetaldehyde oxidation. It has a role as a neuroprotective agent and a spin trapping reagent.
References
1) Nishizawa et al. (2004), Hydroxyl radical generation caused by the reaction of singlet oxygen with the spin trap DMPO, increases significantly in the presence of biological reductants; Mol. Pharmacol., 6 597 2) Shi et al. (2005), Evaluation of spin trapping agents and trapping conditions for detection of cell-generated reactive oxygen species; Arch. Biochem. Biophys., 437 59 3) Clement et al. (2005), Assignment of the EPR spectrum of 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) superoxide spin adduct; J. Org. Chem., 70 1198 4) Gomez-Mejiba et al. (2009), Immuno-spin trapping of protein and DNA radicals: ‘tagging’ free radicals to locate and understand the redox process; Free Rad. Biol. Med., 42 530
Properties of 5,5-DIMETHYL-1-PYRROLINE N-OXIDE
| Melting point: | 25-29 °C (lit.) |
| Boiling point: | 75 °C/0.4 mmHg (lit.) |
| Density | 1.015 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point: | 204 °F |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 25 mg/ml) or in Ethanol (up to 25 mg/ml). |
| form | Pale yellow solid or oil. |
| pka | 2.47±0.40(Predicted) |
| color | Pale yellow |
| Merck | 14,3393 |
| BRN | 107603 |
| Stability: | Stable, but light sensitive, hygroscopic and heat sensitive. Store in the dark, under an inert gas, and keep cold. Incompatible with strong oxidizing agents, moisture. |
| CAS DataBase Reference | 3317-61-1(CAS DataBase Reference) |
Safety information for 5,5-DIMETHYL-1-PYRROLINE N-OXIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P321:Specific treatment (see … on this label). P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 5,5-DIMETHYL-1-PYRROLINE N-OXIDE
| InChIKey | VCUVETGKTILCLC-UHFFFAOYSA-N |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![3-[[2-(BIOTINAMIDO)ETHYL]DITHIO]PROPIONIC ACID 4'-(HYDROXYMETHYL)DMPO ESTER](https://img.chemicalbook.in/CAS/GIF/1255087-87-6.gif)
![5,5-DIMETHYL-1-PYRROLINE, N-OXIDE, [2-14C]-](https://img.chemicalbook.in/StructureFile/ChemBookStructure5/GIF/CB0161594.gif)


You may like
-
 5,5-Dimethyl-1-pyrroline N-Oxide CAS 3317-61-1View Details
5,5-Dimethyl-1-pyrroline N-Oxide CAS 3317-61-1View Details
3317-61-1 -
 5,5-Dimethyl-1-pyrroline N-oxide CAS 3317-61-1View Details
5,5-Dimethyl-1-pyrroline N-oxide CAS 3317-61-1View Details
3317-61-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1