Butyl glycidyl ether
Synonym(s):(Butoxymethyl)oxirane;Araldite RD-1;Butyl 2,3-epoxypropyl ether;Glycidol butyl ether, Butyl glycidyl ether, BGE, 1,2-Epoxy-3-butoxypropane
- CAS NO.:2426-08-6
- Empirical Formula: C7H14O2
- Molecular Weight: 130.18
- MDL number: MFCD00005145
- EINECS: 219-376-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-29 17:45:46
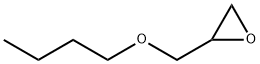
What is Butyl glycidyl ether?
Description
n-Butyl glycidyl ether is a reactive diluent used to reduce viscosity of epoxy res ins Bisphenol A type.
Chemical properties
colourless liquid
Chemical properties
n-Butyl glycidyl ether is a colorless liquid with slight irritating odor.
The Uses of Butyl glycidyl ether
Uses include the roles of viscosity-reducing agent for easier handling of conventional epoxy resins, acid acceptor for stabilizing chlorinated solvents, and chemical intermediate. Some curing agents may produce hazardous polymerizations in large quantities.
The Uses of Butyl glycidyl ether
Viscosity-reducing agent, acid acceptor for solvents, chemical intermediate
Production Methods
n-Butyl glycidyl ether (BGE) is made by the condensation of n-butyl alcohol and epichlorohydrin with subsequent dehydrochlorination with caustic to form the epoxy ring.
General Description
Colorless to pale yellow liquid with a strong, slightly unpleasant odor. Flash point approximately 164°F. Denser than water. Vapors are heavier than air. Vapors may irritate the nose, throat and respiratory tract. Ingestion or inhalation may cause central nervous system depression. Liquid contact may severely irritate the eyes and skin. Prolonged contact with the skin may cause defatting and drying.
Air & Water Reactions
Highly flammable. Butyl glycidyl ether may be sensitive to prolonged exposure to air, may form explosive peroxide in contact with air.
Reactivity Profile
Butyl glycidyl ether, an ether, can act as a base. They form salts with strong acids and addition complexes with Lewis acids. The complex between diethyl ether and boron trifluoride is an example. Ethers may react violently with strong oxidizing agents. In other reactions, which typically involve the breaking of the carbon-oxygen bond, ethers are relatively inert.
Hazard
A mild skin and eye irritant. Sensitization and reproduction effects.
Health Hazard
Exposure can cause mild irritation of skin, eyes, nose, and respiratory tract. Chronic exposure may cause inflammation and sensitization of the skin.
Flammability and Explosibility
Flammable
Contact allergens
A reactive diluent used to reduce viscosity of epoxy resins Bisphenol A type.
Safety Profile
Suspected Carcinogen. Moderately toxic by ingestion, skin contact, and inuaperitoneal routes. Mildly toxic by inhalation. An experimental teratogen. Mutation data reported. A sktn and severe eye irritant. See also ETHERS. When heated to decomposition it emits acrid and irritating fumes.
Potential Exposure
NIOSH has estimated human exposures @ 18,000. Used as reactive diluent for epoxy resins, flooring, laminating, and electrical; and as a stabilizer, viscosity-reducing agent, as acid acceptor for solvents; and as a chemical intermediate
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
Carcinogenicity
BGE was mutagenic in bacterial assays, and DNA damage was induced in human cells in vitro.
Storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with this chemical you should be trainedon its proper handling and storage. Before entering confinedspace where n-butyl glycidyl ether may be present, check tomake sure that an explosive concentration does not exist.Store in a fireproof refrigerator in tightly closed containersunder an inert atmosphere, separated from strong oxidants, strong bases, strong acids. Metal containers involvingthe transfer of this chemical should be grounded andbonded. Where possible, automatically pump liquid fromdrums or other storage containers to process containers.Drums must be equipped with self-closing valves, pressurevacuum bungs, and flame arresters. Use only nonsparkingtools and equipment, especially when opening and closingcontainers of this chemical. Sources of ignition, such assmoking and open flames, are prohibited where this chemical is used, handled, or stored in a manner that could createa potential fire or explosion hazard. A regulated, markedarea should be established where this chemical is handled,used, or stored in compliance with OSHA Standard1910.1045.
Shipping
UN1993 Flammable liquids, n.o.s., Hazard Class: 3; Labels: 3—Flammable liquid, Technical Name Required
Incompatibilities
May form explosive mixture with air. Air and light form unstable and explosive peroxides. Contact with strong oxidizers may cause fire and explosions. Contact with strong caustics may cause polymerization. Attacks some plastics and rubber
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Properties of Butyl glycidyl ether
| Melting point: | 59 °C |
| Boiling point: | 164-166 °C(lit.) |
| Density | 0.91 g/mL at 25 °C(lit.) |
| vapor pressure | 5 hPa (20 °C) |
| refractive index | n |
| Flash point: | 132 °F |
| storage temp. | Store below +30°C. |
| solubility | 20g/l |
| form | Liquid |
| color | Clear colorless |
| Water Solubility | 2 g/100 mL |
| Sensitive | Moisture Sensitive |
| BRN | 103668 |
| Exposure limits | ACGIH: TWA 3 ppm (Skin) OSHA: TWA 50 ppm(270 mg/m3) NIOSH: IDLH 250 ppm; Ceiling 5.6 ppm(30 mg/m3) |
| Stability: | Stable. May form explosive peroxides on contact with air. Store under inert gas. |
| CAS DataBase Reference | 2426-08-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Oxirane, (butoxymethyl)-(2426-08-6) |
| IARC | 2B (Vol. 125) |
| EPA Substance Registry System | n-Butyl glycidyl ether (2426-08-6) |
Safety information for Butyl glycidyl ether
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H226:Flammable liquids H302:Acute toxicity,oral H314:Skin corrosion/irritation H317:Sensitisation, Skin H331:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H341:Germ cell mutagenicity H350:Carcinogenicity H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Butyl glycidyl ether
Butyl glycidyl ether manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Butyl Glycidyl Ether CAS 2426-08-6View Details
Butyl Glycidyl Ether CAS 2426-08-6View Details
2426-08-6 -
 Butyl 2,3-epoxypropyl ether CAS 2426-08-6View Details
Butyl 2,3-epoxypropyl ether CAS 2426-08-6View Details
2426-08-6 -
 Butyl glycidyl ether CAS 2426-08-6View Details
Butyl glycidyl ether CAS 2426-08-6View Details
2426-08-6 -
 Butyl Glycidyl Ether, LiquidView Details
Butyl Glycidyl Ether, LiquidView Details
2426-08-6 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
