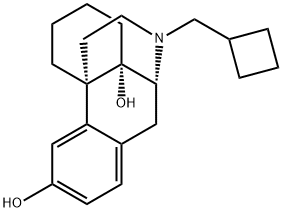
Butorphanol tartrate
Synonym(s):L -N-Cyclobutylmethyl-3,14-dihydroxymorphinan (+)-tartrate salt;Butorphanol (+)-tartrate salt
- CAS NO.:58786-99-5
- Empirical Formula: C25H35NO8
- Molecular Weight: 477.55
- MDL number: MFCD00214257
- EINECS: 261-443-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-20 16:56:08
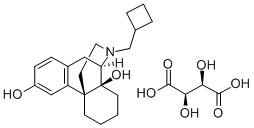
What is Butorphanol tartrate?
Description
Butorphanol (tartrate) (Item No. 23812) is an analytical reference material categorized as an opioid. Butorphanol is regulated as a Schedule IV compound in the United States. This product is intended for research and forensic applications.
The Uses of Butorphanol tartrate
Analgesic; antitussive.
Definition
ChEBI: The (S,S)-tartaric acid salt of butorphanol. It is used for relief or moderate to severe pain.
brand name
Stadol (Bristol-Myers Squibb).
Biochem/physiol Actions
κ/μ opioid receptor agonist.
Mechanism of action
Butorphanol is a strong agonist at κ opioid receptors, and through this interaction, it is five times more potent than morphine as an analgesic. The κagonists have a lower ceiling analgesic effect than full μ agonists; thus, they are not as effective in treating severe pain. Butorphanol is an antagonist at μ opioid receptors with approximately one-sixth the potency of naloxone. If given to a person addicted to a μ agonist, butorphanol will induce an immediate onset of abstinence syndrome.
Side Effects
Butorphanol has a different spectrum of side effects than μ opioid analgesics. Respiratory depression occurs. There is a lower ceiling on this effect, however, and it is not generally lethal, as is the case with high doses of μ agonists. Major side effects after normal analgesic doses are sedation, nausea, and sweating, as well as dysphoric (hallucinogenic) effects at higher doses. Butorphanol causes an increase in pulmonary arterial pressure and pulmonary vascular resistance. There is an overall increased workload on the heart, and it should not be used in patients with congestive heart failure or to treat pain from acute myocardial infarction. Butorphanol has low abuse potential and is not a scheduled drug.
Veterinary Drugs and Treatments
Approved indication for dogs is “. . . for the relief of chronic nonproductive
cough associated with tracheobronchitis, tracheitis,
tonsillitis, laryngitis and pharyngitis originating from inflammatory
conditions of the upper respiratory tract” (Package Insert;
Torbutrol?—Fort Dodge). It is also used in practice in both dogs and
cats as a preanesthetic medication, analgesic, and as an antiemetic
prior to cisplatin treatment (although not very effective in cats for
this indication). Compared with other opiate analgesics, butorphanol
is not very useful in small animals (particularly dogs) for treating
pain and has to be dosed frequently.
The approved indication for horses is “. . . for the relief of pain
associated with colic in adult horses and yearlings” (Package Insert;
Torbugesic?—Fort Dodge). It has also been used clinically as an analgesic
in cattle.
Properties of Butorphanol tartrate
| Melting point: | 217-219° |
| alpha | D22 -64.0° (c = 0.4 in methanol) |
| storage temp. | 2-8°C |
| CAS DataBase Reference | 58786-99-5(CAS DataBase Reference) |
Safety information for Butorphanol tartrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H361:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Butorphanol tartrate
Butorphanol tartrate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
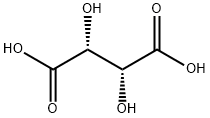

You may like
-
 58786-99-5 Butorphanol tartrate 98%View Details
58786-99-5 Butorphanol tartrate 98%View Details
58786-99-5 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
