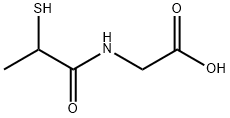
BUCILLAMINE
- CAS NO.:65002-17-7
- Empirical Formula: C7H13NO3S2
- Molecular Weight: 223.31
- MDL number: MFCD00867570
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
What is BUCILLAMINE?
Description
Bucillamine is an orally active immunomodulator useful in the treatment of rheumatoid arthritis. Its potencies in inhibiting collagenase activity and inactivating rheumatoid factor far exceed those of penicillamine. In man, bucillamine reportedly brings about significant improvements over placebo in erythrocyte sedimentation, grip power, joint swelling and duration of morning stiffness.
Chemical properties
Off-White Solid
Originator
Santen (Japan)
The Uses of BUCILLAMINE
An antirheumatic agent.
What are the applications of Application
Bucillamine is an antirheumatic agent
Definition
ChEBI: Bucillamine is an organic molecular entity.
Manufacturing Process
Preparation of N-(2-benzylmercaptoisobutyryl)-S-benzyl-L-cysteine:
1). 73.9 g of S-benzyl-L-cysteine were dissolved in 700 ml of 1 N sodium
hydroxide solution. The solution was cooled in an ice bath and stirred. 2-
Benzylmercaptoisobutyryl chloride, which was obtained by reacting 63.1 g of
2-benzylmercaptoisobutyric acid with 39.3 g of thionyl chloride, was added
dropwise to this solution. The resulting mixture was then stirred for one hour,
acidified with hydrochloric acid and extracted with ethyl acetate. The extract
was washed with water, dried over sodium sulfate and evaporated to dryness.
The residue was chromatographed on silica gel with benzene/ethylacetate
(1:1) as an eluant. The eluate was evaporated to dryness and an oily residue weighing 46.9 g, representing a yield of 74%, was obtained.
2). The obtained in (1) above were dissolved in 500 ml of liquid ammonia and
21.1 g of metallic sodium were added slowly with stirring. After completion of
reaction, 59.4 g of ammonium chloride were added and thereafter the
ammonia was removed by distillation. Water was added to the residue to
dissolve the solid. The resulting water layer was separated, washed with ethyl
acetate, and acidified with hydrochloric acid under cooling. The precipitates
thus obtained were extracted with ethyl acetate. The extract was washed with
water, dried over sodium sulfate and evaporated to dryness. The product
weighed 43.6 g, representing a yield of 88%. After recrystallization from ethyl
acetate, the desired compound, melting at 139°-140°C, was obtained.
[α]D
25=+32.3° (c=1.0, ethanol).
brand name
Rimatil
Therapeutic Function
Antirheumatic, Immunomodulator
Properties of BUCILLAMINE
| Melting point: | 119-123°C |
| Boiling point: | 438.0±45.0 °C(Predicted) |
| alpha | D25 +32.3° (c = 1.0 in ethanol) |
| Density | 1.3934 (rough estimate) |
| refractive index | 1.6370 (estimate) |
| storage temp. | Hygroscopic, -20°C Freezer, Under Inert Atmosphere |
| solubility | DMSO (Very Slightly), Ethanol (Slightly), Methanol (Slightly), Water (Slightly) |
| pka | 3.01±0.10(Predicted) |
| form | Solid |
| color | White to Pale Yellow |
| Stability: | Air Sensitive, Hygroscopic, Unstable in Solution |
| CAS DataBase Reference | 65002-17-7(CAS DataBase Reference) |
Safety information for BUCILLAMINE
Computed Descriptors for BUCILLAMINE
BUCILLAMINE manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Bucillamine 99%View Details
Bucillamine 99%View Details -
 Bucillamine 95% CAS 65002-17-7View Details
Bucillamine 95% CAS 65002-17-7View Details
65002-17-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8