BroModifluoroMethyl phenyl sulfone
- CAS NO.:80351-58-2
- Empirical Formula: C7H5BrF2O2S
- Molecular Weight: 271.08
- MDL number: MFCD22628354
- Update Date: 2024-04-15 18:41:30
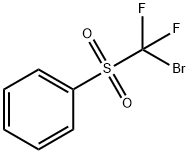
What is BroModifluoroMethyl phenyl sulfone?
Description
The nucleophilic reactions of bromodifluoromethyl phenyl sulfone with electrophiles such as aldehydes in the presence of TDAE affords (phenylsulfonyl)difluoromethyl-containing synthetically useful intermediates. Palladium-mediated reactions of styrene derivatives, vinyl ethers, and heteroaromatics with bromodifluoromethyl phenyl sulfone in the presence of potassium carbonate affords the (phenylsulfonyl)difluoromethylated products.
The Uses of BroModifluoroMethyl phenyl sulfone
Bromodifluoromethyl Phenyl Sulfone can be used to prepare difluoromethyl ethers via difluoromethylation of alcohols.
The Uses of BroModifluoroMethyl phenyl sulfone
Bromodifluoromethyl phenyl sulfone is an intermediate component in the synthesis of (phenylsulfonyl)difluoromethyl and can be used to prepare (phenylsulfonyl)difluoromethylated products by palladium-mediated reactions.
Reactions
(1) Difluoromethylation of aldehydes.
(2) Difluoromethylation of styrenes, vinyl ethers, and heteroaromatics.
References
[1] G.K. SURYA PRAKASH . Nucleophilic difluoromethylation and difluoromethylenation using bromodifluoromethyl phenyl sulfone[J]. Journal of Fluorine Chemistry, 2005. DOI:10.1016/j.jfluchem.2005.07.011.
[2] NAKIN SURAPANICH. ChemInform Abstract: Palladium-Mediated Heck-Type Reactions of [(Bromodifluoromethyl)sulfonyl]benzene: Synthesis of α-Alkenyl- and α-Heteroaryl-Substituted α,α-Difluoromethyl Phenyl Sulfones.[J]. ChemInform, 2013. DOI:10.1002/chin.201314090.
Properties of BroModifluoroMethyl phenyl sulfone
| Melting point: | 33-34 °C |
| Boiling point: | 291.8±40.0 °C(Predicted) |
| Density | 1.745±0.06 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | Chloroform (Sparingly), DMSO (Slightly) |
| form | Solid |
| color | White to Off-White Low-Melting |
Safety information for BroModifluoroMethyl phenyl sulfone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for BroModifluoroMethyl phenyl sulfone
New Products
4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION Methylcobalamin (vitamin B12) SODIUM METHYL PARABEN SODIUM VALPROATE AMOXICILLIN (AMOXYCILLIN) TRIHYDRATE ACICLOVIRRelated products of tetrahydrofuran
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 1714107-48-8 5-Iodo-3-methyl-2-nitropyridine 98%View Details
1714107-48-8 5-Iodo-3-methyl-2-nitropyridine 98%View Details
1714107-48-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9