Bromoacetic acid
Synonym(s):α-Bromoacetic acid;2-Bromoethanoic acid;Bromoacetic acid;Monobromoacetic acid
- CAS NO.:79-08-3
- Empirical Formula: C2H3BrO2
- Molecular Weight: 138.95
- MDL number: MFCD00002678
- EINECS: 201-175-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
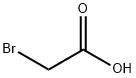
What is Bromoacetic acid?
Chemical properties
WHITE TO LIGHT YELLOW CRYSTALLINE MASS
The Uses of Bromoacetic acid
Bromoacetic acid is used in organic synthesis and as an alkylating agent. It is also used as a biochemical for proteomics research. It is an important raw material and intermediate used in organic synthesis, pharmaceuticals, dyes and agrochemicals.
The Uses of Bromoacetic acid
Organic synthesis, abscission of citrus fruit in harvesting.
The Uses of Bromoacetic acid
Bromoacetic acid is mainly used for the N-terminal bromoacylation of resin bound peptides.
It can also be used:
- To synthesize (Z)-2-(cyclooct-4-en-1-yloxy)acetic acid.
- To synthesize α-bromo-phenylacetamide.
- To convert aromatic thiosemicarbazones to thiazolylhydrazones.
What are the applications of Application
Bromoacetic acid is used in organic synthesis
General Description
Aqueous solution.
Air & Water Reactions
Water soluble.
Reactivity Profile
Carboxylic acids, such as BROMOACETIC ACID, donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Bromoacetic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions.
Hazard
Strong irritant to skin and tissue.
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Contact with molten substance may cause severe burns to skin and eyes. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by ingestion, intraperitoneal, and intravenous routes. Irritating and corrosive to skin and mucous membranes. Mutation data reported. When heated to decomposition it emits toxic fumes of Br-. See also BROMIDES.
Purification Methods
Crystallise bromoacetic acid from pet ether (b 40-60o). A diethyl ether solution of it is passed through an alumina column, and the ether is evaporated at room temperature under vacuum. It is best obtained by distillation from a Claisen (flask immersed in an oil bath) fitted with an insulated Vigreux column (p 11) and the fraction b 108-110o/30mm is collected. It is light and moisture sensitive. [Natelson & Gottfried Org Synth Coll Vol III 381 1955, Beilstein 2 IV 526.] LACHRYMATORY and is a skin IRRITANT.
Properties of Bromoacetic acid
| Melting point: | 46-49 °C |
| Boiling point: | 208 °C(lit.) |
| Density | 1 |
| vapor pressure | 1 mm Hg @ 550C |
| refractive index | 1.4804 (estimate) |
| Flash point: | >110°C |
| storage temp. | Store below +30°C. |
| solubility | chloroform: soluble10%, clear, colorless |
| pka | 2.69(at 25℃) |
| form | Crystalline Mass or Crystalline Powder, Flakes, Crystals and/or Chunks |
| color | White to yellow |
| Water Solubility | miscible |
| Sensitive | Light Sensitive & Hygroscopic |
| Merck | 14,1400 |
| BRN | 506167 |
| CAS DataBase Reference | 79-08-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetic acid, bromo-(79-08-3) |
| EPA Substance Registry System | Bromoacetic acid (79-08-3) |
Safety information for Bromoacetic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H317:Sensitisation, Skin H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Bromoacetic acid
Bromoacetic acid manufacturer
Sibram Pharmaceutical
S D Fine Chem Limited
Related products of tetrahydrofuran

![Acetamide, 2-[4-[(5,6-diphenyl-2-pyrazinyl)(1-methylethyl)amino]butoxy]-](https://img.chemicalbook.in/CAS/20210111/GIF/475086-28-3.gif)
![Acetamide, 2-[4-[(1-methylethyl)amino]butoxy]-N-(methylsulfonyl)-](https://img.chemicalbook.in/CAS/20210111/GIF/2010956-45-1.gif)
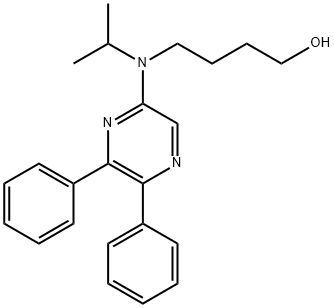
![[4-[(5,6-DIPHENYLPYRAZINYL)(1-METHYLETHYL)AMINO]BUTOXY]-ACETIC ACID](https://img.chemicalbook.in/CAS2/GIF/475085-57-5.gif)
You may like
-
 Bromoacetic acid 79-08-3 99%View Details
Bromoacetic acid 79-08-3 99%View Details
79-08-3 -
 Bromoacetic Acid CAS 79-08-3View Details
Bromoacetic Acid CAS 79-08-3View Details
79-08-3 -
 Bromoacetic Acid pure CAS 79-08-3View Details
Bromoacetic Acid pure CAS 79-08-3View Details
79-08-3 -
 Bromoacetic acid 95% CAS 79-08-3View Details
Bromoacetic acid 95% CAS 79-08-3View Details
79-08-3 -
 Bromoacetic acid >99% (GC) CAS 79-08-3View Details
Bromoacetic acid >99% (GC) CAS 79-08-3View Details
79-08-3 -
 Bromoacetic acid CAS 79-08-3View Details
Bromoacetic acid CAS 79-08-3View Details
79-08-3 -
 BROMOACETIC ACID For Synthesis CAS 79-08-3View Details
BROMOACETIC ACID For Synthesis CAS 79-08-3View Details
79-08-3 -
 Bromoacetic acid solution CAS 79-08-3View Details
Bromoacetic acid solution CAS 79-08-3View Details
79-08-3