Boron triiodide
- CAS NO.:13517-10-7
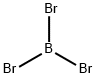
- Empirical Formula: BI3
- Molecular Weight: 391.52
- MDL number: MFCD00036286
- EINECS: 236-857-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
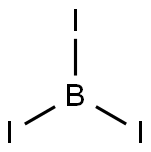
What is Boron triiodide?
Chemical properties
needles or crystal(s) with 99.9% purity; unstable; enthalpy of vaporization 40.5 kJ/mol. It is a crystalline solid, which reacts vigorously with water to form hydroiodic acid and boric acid. Boron triiodide is easily decomposed when heated, and reacts with red phosphorus or white phosphorus to incandescent.
Boron triiodide is an electron-deficient compound with unsaturated boron atoms, so its chemical properties are mainly Lewis acid properties, and it can react with various Lewis bases to form complexes.
The Uses of Boron triiodide
Boron triiodide is used as pharmaceutical, chemical and organic intermediate. It is also used as a catalyst and for preparation of boron compounds.
The Uses of Boron triiodide
Boron triiodide can be used as a reagent to cleave C-O bonds in ethers, esters, and alcohols. It can also be used to cleave silanes and halides. Boron triiodide converts alcohols to alkyl iodides; sulfonyl and sulfinyl to disulfides. Borylation of triarylamines can be achievd using this reagent.
It can also be used to prepare imidazole based imino aluminum dihalide metal complexes and boron carbonitride (BCN) nanowires.
Preparation
Boron triiodide can be synthesized by refluxing lithium borohydride and iodine in hexane, or by reacting alkali metal borohydride with iodine at a suitable temperature.
General Description
Boron triiodide is a strong Lewis acid. It can be prepared by treating iodine (I2) with potassium borohydride (KBH4) in heptane.
Properties of Boron triiodide
| Melting point: | 43-44°C |
| Boiling point: | 210°C |
| Density | 3.35 g/mL at 25 °C (lit.) |
| Flash point: | 210°C |
| storage temp. | 2-8°C |
| solubility | insoluble in H2O |
| form | Crystals |
| color | White |
| Specific Gravity | 3.35 |
| Water Solubility | Reacts with water. |
| Sensitive | Moisture Sensitive |
| CAS DataBase Reference | 13517-10-7(CAS DataBase Reference) |
| EPA Substance Registry System | Borane, triiodo- (13517-10-7) |
Safety information for Boron triiodide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Boron triiodide
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Boron triiodide CAS 13517-10-7View Details
Boron triiodide CAS 13517-10-7View Details
13517-10-7 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1