Bladex
- CAS NO.:21725-46-2
- Empirical Formula: C9H13ClN6
- Molecular Weight: 240.69
- MDL number: MFCD00055514
- EINECS: 244-544-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
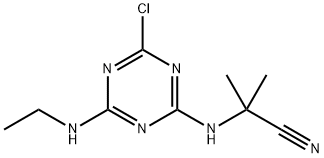
What is Bladex?
Description
Cyanazine is an off-white to tan crystallinesolid. Molecular weight=240.73; Freezing/Meltingpoint=167-169℃. Hazard Identification (based on NFPA-704 M Rating System): Health 2, Flammability 1,Reactivity 0. Soluble in water. Physical properties may bealtered by carrier solvents used in commercial formulations
Chemical properties
Cyanazine is an off-white to tan crystalline solid. Molecular . Hazard identification (based on NFPA-704 M Rating System): Health 2, flammability 1, reactivity 0. Soluble in water. Physical properties may be altered by car- rier solvents used in commercial formulations.
The Uses of Bladex
Selective pre-emergence herbicide.
The Uses of Bladex
Selective pre- and postemergence herbicide to control weeds in crops such as alfalfa, corn, cotton, sorghum, soybeans, and wetland.
The Uses of Bladex
Herbicide used for control of annual grasses and broad-leaved weeds in cereals, cotton, maize, onions, peanuts, peas, potatoes, soybeans, sugarcane and wheat fallow
Definition
ChEBI: Cyanazine is a chloro-1,3,5-triazine that is 2-chloro-1,3,5-triazine substituted by an ethyl amino and a (2-cyanopropan-2-yl)amino group at positions 6 and 4 respectively. It has a role as a herbicide, an environmental contaminant and a xenobiotic. It is a 1,3,5-triazinylamino nitrile and a chloro-1,3,5-triazine.
General Description
Colorless crystals. Non corrosive when dry. Used as a selective systemic herbicide.
Air & Water Reactions
Stable at pHs between 5.0 and 9.0, but is hydrolyzed by strong acids and bases.
Reactivity Profile
A triazine derivative.
Hazard
Very toxic.
Agricultural Uses
Herbicide: On August 2, 1995, the U.S. EPA announced a voluntary phase-out of the use of cyanazine. On January 6, 2000, the U.S. EPA announced the cancellation of all cyanazine registrations. It had been A U.S. EPA restricted Use Pesticide (RUP) because of its teratogenicity and because it has been found in groundwater. Not approved for use in EU countries. Cyanazine is used as a pre-and post-emergent herbicide to control annual grasses and broadleaf weeds. It is used mostly on corn, some on cotton, and less than 1% on grain sorghum and wheat fallow. The compound is formulated as a wettable powder, a flowable suspension, or as granules. In California, major usages are on cotton, corn and corn for forage, almonds, and uncultivated agricultural areas. In 1995, cyanazine was the fourth most widely used synthetic pesticide in the U.S. Cyanazine, atrazine, and simazine are collectively referred to as the triazines and may be alternatively used for each other on corn and in some other situations.
Trade name
BLADEX®[C]; BLADEX® 80WP[C]; BULLET®; CYCLE®; CY-PRO®[C]; CYNEX® 41[C]; DW 3418®; EXTRAZINE®[C]; FORTROL®; MATCH®; PAYZE®; SD 15418®; WL 19805®
Potential Exposure
A potential danger to those involved in the manufacture, formulation, and application of this her- bicide used for preemergence or postemergence weed con- trol in field corn.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Carcinogenicity
No increase in tumor incidence was noted in rats fed up to 50 ppm of cyanazine for 2 years. The same was true for mice given up to 1000 ppm in their diets for 2 years.
Environmental Fate
Soil. In sandy loam soils (foc = 0.01), the half-life is 12–15 days. However, in silt loam
(foc = 0.028) and clay loam (foc = 0.03) soils, the reported half-life is 20–25 days (Humburg
et al., 1989). The half-life of cyanazine is longer in alkaline soils (pH >7.5) than in acidic
soils (pH <5.5) (Humburg et al., 1989)
Groundwater. According to the U.S. EPA (1986) cyanazine has a high potential to
leach to groundwater. Cyanazine amide was identified as a metabolite in groundwater in
corn fields (Muir and Baker, 1976)
Plant. In plants, cyanazine is degraded by elimination of the ethyl group, hydration
of the cyano group and the removal and replacement of the chlorine atom by a hydroxyl
group (Humburg et al., 1989).
Chemical/Physical. In laboratory tests, the nitrile group was hydrolyzed to the corresponding carboxylic acid. The rate of hydrolysis is faster under higher temperatures and
low pHs (Grayson, 1980). The chlorine atom may be replaced by a hydroxyl group forming
2((4-hydroxy-6-(ethylamino)-1,3,5-triazin-2-yl)amino)-2-methylpropanenitrile (Hartley
and Kidd, 1987)
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with cyanazineyou should be trained on its proper handling and storage.Store in tightly closed containers in a cool, well-ventilatedarea away from heat. Where possible, automatically pumpliquid from drums or other storage containers to processcontainers.
Shipping
UN2763 Triazine pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN3439 Nitriles, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1- Poisonous materials, Technical Name Required.
Incompatibilities
Cyanazine decomposes in heat producing very toxic fumes and gases of hydrogen cyanide, hydrogen chloride; ethyl chloride; ammonia; acetone; and ethylene. Nitriles may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cya- nide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are Soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids .Attacksmetals in the presence of heat and moisture.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinera- tor equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. In accordance with 40CFR165, follow recom- mendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following pack- age label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Properties of Bladex
| Melting point: | 167°C |
| Boiling point: | 383.12°C (rough estimate) |
| Density | 1.2870 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| Flash point: | 100 °C |
| storage temp. | APPROX 4°C
|
| solubility | DMSO: 100 mg/mL (415.47 mM) |
| form | Granular Powder |
| pka | 1.46±0.41(Predicted) |
| color | Off-white |
| Water Solubility | 0.0171 g/100 mL |
| Merck | 13,2714 |
| BRN | 615509 |
| CAS DataBase Reference | 21725-46-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Cyanazine(21725-46-2) |
| EPA Substance Registry System | Cyanazine (21725-46-2) |
Safety information for Bladex
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. |
Computed Descriptors for Bladex
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 3-NITRO-2-METHYL ANILINE 4-IODO BENZOIC ACID 4-HYDROXY BENZYL ALCOHOL 4-(3-chloropropyl)morpholine phenylhydrazine hydrochloride (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamateRelated products of tetrahydrofuran




You may like
-
 Cyanazine 99%View Details
Cyanazine 99%View Details -
 (9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
(9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
82911-69-1 -
 13057-17-5 95.0%View Details
13057-17-5 95.0%View Details
13057-17-5 -
 4-bromoaniline 106-40-1 99.0%View Details
4-bromoaniline 106-40-1 99.0%View Details
106-40-1 -
 1421517-99-8 99.0%View Details
1421517-99-8 99.0%View Details
1421517-99-8 -
 5-bromo-2-chlorobenzoic acid 99.0%View Details
5-bromo-2-chlorobenzoic acid 99.0%View Details
21739-92-4 -
 2-methyl-5-nitrophenol 98.0%View Details
2-methyl-5-nitrophenol 98.0%View Details
5428-54-6 -
 15761-38-3 97.0%View Details
15761-38-3 97.0%View Details
15761-38-3