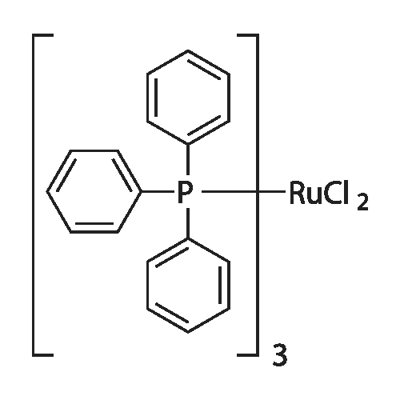
Bis(triphenylphosphine)palladium(II) chloride
Synonym(s):Dichlorobis(triphenylphosphine)palladium(II);Palladium(II)bis(triphenylphosphine) dichloride;PdCl2(PPh3)2;PdCl2(PPh3)2 impregnated tablets;Bis(triphenylphosphine)palladium(II) chloride (15.2% Pd)
- CAS NO.:13965-03-2
- Empirical Formula: C36H30Cl2P2Pd
- Molecular Weight: 701.9
- MDL number: MFCD00009593
- EINECS: 237-744-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
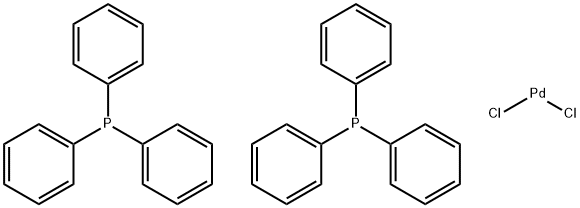
What is Bis(triphenylphosphine)palladium(II) chloride?
Description
Pd(PPh3)2Cl2 is a catalyst commonly employed in palladium catalyzed reactions such as Suzuki reactions, Sonogashira reactions, Heck reactions, etc. Pd(PPh3)2Cl2 is generally more stable at room temperature and has a longer shelf-life than Pd(PPh3)4.
Chemical properties
Bis(triphenylphosphine)palladium chloride is a coordination compound of palladium containing two triphenylphosphine and two chloride ligands. This yellow solid is insoluble in water but can dissolve in some organic solvents, such as toluene and benzene, and is slightly soluble in acetone and chloroform. It is used for palladium-catalyzed coupling reactions, e.g. the Sonogashira–Hagihara reaction. The complex adopts a square planar geometry and numerous similar complexes are known, with different phosphine ligands.
The Uses of Bis(triphenylphosphine)palladium(II) chloride
Bis(triphenylphosphine)palladium(II) chloride is used primarily in organometallic catalytic reactions due to the palladium content of the compound. It is also involved in crystalized structures of me tallacyclic complexes which show antiinflammatory and antifungal properties.
Synthesis
The synthesis of Bis(triphenylphosphine)palladium(II) chloride can be achieved by the reaction of palladium(II) chloride with triphenylphosphine, resulting in the formation of Bis(triphenylphosphine)palladium(II) chloride. This chemical process is represented by the balanced equation: PdCl2 + 2 PPh3 → PdCl2(PPh3)2.
Reactions
Precatalyst for the carbonylative cyclization of malonate derivatives.
Catalyst used in the double allylation of activated olefins.
Precatalyst for the three-component preparation of 3-arylidene- (or 3-alkenylidene) tetrahydrofurans.
Precatalyst for the homocoupling of terminal alkynes.
Precatalyst in the cross-coupling of alkynylsilanols and aryl halides.
Catalyst for direct Pd-catalyzed alkynylation of N-fused heterocycles.
Catalyst for a tandem Heck reaction/C-H functionalization.
Catalyst for direct arylation of tautomerizable heterocycles. 


General Description
Bis(triphenylphosphine)palladium(II) dichloride is an organometallic complex. It is an efficient cross-coupling catalyst for C-C coupling reaction, such as Negishi coupling, Suzuki coupling, Sonogashira coupling and Heck coupling reaction. Detection of bis(triphenylphosphine)palladium(II) dichloride by electrospray ionization quadrupole ion trap mass spectrometry using different imidazolium salts as the charge carrier has been reported. It is employed as catalyst for the Heck reaction medium.
Flammability and Explosibility
Non flammable
Structure
Several crystal structures containing PdCl2(PPh3)2 have been reported. In all of the structures, PdCl2(PPh3)2 adopts a square planar coordination geometry and the trans isomeric form.
Preparation
The Iodo compound (308 mg, 0.513 mmol), the boronic ester (194 mg, 0.641 mmol), K3PO4 (327 mg, 1.539 mmol), XPhos (14.67 mg, 0.031 mmol), and Pd2(dba)3 (14.09 mg, 0.015 mmol) were combined in a microwave vial. The vial was purged with argon, after which time was added dioxane (3 mL) and H2O (0.5 mL) The reaction was irradiated in a microwave reactor at 120 C for 10 min. Additional boronic ester (50 mg) was added and the mixture was irradiated at 120 C for another 10 min. The mixture was then treated with aq 1N NaOH and extracted with EtOAc (50 mL). The org layer was dried (MgSO4), concentrated, and purified by flash chromatography (20% MeOH/DCM) to provide the product as a dark yellow solid. [177 mg, 53%]
Properties of Bis(triphenylphosphine)palladium(II) chloride
| Melting point: | 260°C |
| vapor pressure | 0Pa at 25℃ |
| RTECS | RT3578000 |
| storage temp. | Store below +30°C. |
| solubility | Chloroform (Slightly), Dichloromethane (Slightly, Heated), Methanol (Slightly, |
| appearance | Yellow solid |
| form | Powder |
| color | Yellow |
| Water Solubility | Insoluble in water. Soluble in benzene, and toluene. |
| Sensitive | Hygroscopic |
| BRN | 4935975 |
| InChI | InChI=1S/2C18H15P.2ClH.Pd/c2*1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;;;/h2*1-15H;2*1H;/q;;;;+2/p-2 |
| CAS DataBase Reference | 13965-03-2(CAS DataBase Reference) |
Safety information for Bis(triphenylphosphine)palladium(II) chloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for Bis(triphenylphosphine)palladium(II) chloride
| InChIKey | ILBDOZRDKNIJBS-UHFFFAOYSA-N |
| SMILES | P(C1C=CC=CC=1)(C1=CC=CC=C1)C1C=CC=CC=1.P(C1C=CC=CC=1)(C1C=CC=CC=1)C1C=CC=CC=1.[Pd](Cl)Cl |
Bis(triphenylphosphine)palladium(II) chloride manufacturer
JSK Chemicals
Vineeth Precious Catalysts Pvt. Ltd.
Omkar Lab
ASM Organics
New Products
Tetrabutylammonium hydrogen sulfate 2,2,6-Trimethyl-4H-1,3-dioxin-4-one 4-Piperidinopiperidine tert-Butyl acetoacetate 4-BROMOMETHYLTETRAHYDROPYRAN 3-Hydroxyazetidine hydrochloride Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) 3-Methoxybenzonitrile 4-Methoxybenzonitrile Dibenzoyl Peroxide Titanium Dioxide Chloral PentachlorobenzonitrileRelated products of tetrahydrofuran



You may like
-
 Bis(triphenylphosphine)palladium(II)chloride 99%View Details
Bis(triphenylphosphine)palladium(II)chloride 99%View Details -
 13965-03-2 98%View Details
13965-03-2 98%View Details
13965-03-2 -
 trans-Dichlorobis(triphenylphosphine)palladium(II) CAS 13965-03-2View Details
trans-Dichlorobis(triphenylphosphine)palladium(II) CAS 13965-03-2View Details
13965-03-2 -
 trans-Dichlorobis(triphenylphosphine)palladium(II) CAS 13965-03-2View Details
trans-Dichlorobis(triphenylphosphine)palladium(II) CAS 13965-03-2View Details
13965-03-2 -
 trans-Dichlorobis(triphenylphosphine)palladium(II) CAS 13965-03-2View Details
trans-Dichlorobis(triphenylphosphine)palladium(II) CAS 13965-03-2View Details
13965-03-2 -
 Bis(triphenylphosphine)palladium(II) dichloride CAS 13965-03-2View Details
Bis(triphenylphosphine)palladium(II) dichloride CAS 13965-03-2View Details
13965-03-2 -
 Dichlorobis(triphenylphosphine)palladium(II) 98% CAS 13965-03-2View Details
Dichlorobis(triphenylphosphine)palladium(II) 98% CAS 13965-03-2View Details
13965-03-2 -
 Dichlorobis(triphenylphosphine)palladium(ii) CAS 13965-03-2View Details
Dichlorobis(triphenylphosphine)palladium(ii) CAS 13965-03-2View Details
13965-03-2
