Bis(2-ethylhexyl) phosphate
Synonym(s):Bis(2-ethylhexyl) hydrogen phosphate;Bis(2-ethylhexyl) phosphate;HDEHP;Di(2-ethylhexyl) phosphate;HDEHP, Phosphoric acid bis (2-ethylhexyl) ester, Bis(2-ethylhexyl) phosphoric acid
- CAS NO.:298-07-7
- Empirical Formula: C16H35O4P
- Molecular Weight: 322.42
- MDL number: MFCD00009492
- EINECS: 206-056-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-29 10:02:07
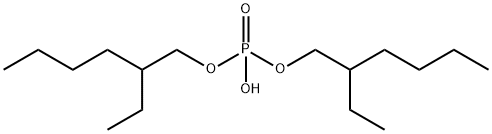
What is Bis(2-ethylhexyl) phosphate?
Chemical properties
clear colorless to light yellow liquid
The Uses of Bis(2-ethylhexyl) phosphate
Extraction of metals.Bis(2-ethylhexyl) phosphate is used as a lubricant additive, corrosion inhibitor and a metal extractant. It is involved in the solvent extraction of uranium salts and rare earth metals. Iron(II) ions are extracted after reduction of Iron(III) ions. Further, it is used as a plasticizer and as a solvent in the synthesis of plastic.
The Uses of Bis(2-ethylhexyl) phosphate
Additive to lubrication oils, corrosion inhibitor, and antioxidant. Used as extractant in the hydrometallurgical separation of cobalt and nickel. Metal extraction and separation, intermediate for wetting agents and detergents . Feedstock for chemical synthesis; extraction fluid for metal salts; cation extracting agent.
Production Methods
Produced by chlorinating bis(2-ethylhexyl) phosphonate to give the phosphate diester chloride, followed by hydrolysis, or by saponification of tris(2-ethylhexyl) phosphate .
Synthesis
2-ethylhexan-1-ol could be used as a starting material to synthesize Bis(2-ethylhexyl) phosphate.
General Description
Odorless light yellow liquid. Floats on water.
Reactivity Profile
Organophosphates, such as Bis(2-ethylhexyl) phosphate, are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides. Mildly corrosive to most metals; may form flammable hydrogen gas [USCG, 1999].
Health Hazard
Contact with liquid irritates eyes and may cause serious injury; consult an eye specialist. Also causes skin irritation on contact. Ingestion produces irritation similar to that caused by strong vinegar.
Fire Hazard
Special Hazards of Combustion Products: Irritating phosphorus oxides may be released.
Flammability and Explosibility
Non flammable
Purification Methods
Contaminants of commercial samples include the monoester, polyphosphates, pyrophosphate, 2-ethylhexanol and metal impurities. Dissolve the acid in n-hexane to give an 0.8M solution. Wash this with an equal volume of M HNO3, then with saturated (NH4)2CO3 solution, with 3M HNO3, and twice with water [Petrow & Allen Anal Chem 33 1303 1961]. Similarly, the impure sodium salt, after scrubbing with pet ether, is acidified with HCl and the free organic acid is extracted into pet ether and purified as above [Peppard et al. J Inorg Nucl Chem 7 231 1958], or as described by Stewart & Crandall [J Am Chem Soc 73 1377 1951]. It can be purified via its copper salt [McDowell et al. J Inorg Nucl Chem 38 2127 1976]. [Beilstein 1 IV 1796.]
Properties of Bis(2-ethylhexyl) phosphate
| Melting point: | −60 °C(lit.) |
| Boiling point: | 48 °C (12 mmHg) |
| Density | 0.965 g/mL at 25 °C(lit.) |
| vapor pressure | <0.1 hPa (20 °C) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | ethanol: soluble100mg/mL, clear |
| form | Liquid |
| pka | 1.47±0.50(Predicted) |
| color | Colorless |
| Specific Gravity | 0.974 |
| PH | 3 (< 1g/l, H2O) |
| Water Solubility | slightly soluble |
| FreezingPoint | -60℃ |
| BRN | 1712988 |
| Stability: | Moisture Sensitive |
| CAS DataBase Reference | 298-07-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Bis(2-ethylhexyl)hydrogen phosphate(298-07-7) |
| EPA Substance Registry System | Bis(2-ethylhexyl) phosphate (298-07-7) |
Safety information for Bis(2-ethylhexyl) phosphate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H312:Acute toxicity,dermal H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Bis(2-ethylhexyl) phosphate
| InChIKey | SEGLCEQVOFDUPX-UHFFFAOYSA-N |
Bis(2-ethylhexyl) phosphate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Bis-(2-ethylhexyl)-phosphate 98%View Details
Bis-(2-ethylhexyl)-phosphate 98%View Details -
 Bis(2-ethylhexyl) phosphate CAS 298-07-7View Details
Bis(2-ethylhexyl) phosphate CAS 298-07-7View Details
298-07-7 -
 Bis(2-ethylhexyl) phosphate CAS 298-07-7View Details
Bis(2-ethylhexyl) phosphate CAS 298-07-7View Details
298-07-7 -
 Bis(2-ethylhexyl) Phosphate CAS 298-07-7View Details
Bis(2-ethylhexyl) Phosphate CAS 298-07-7View Details
298-07-7 -
 Di(2-ethylhexyl) phosphate CAS 298-07-7View Details
Di(2-ethylhexyl) phosphate CAS 298-07-7View Details
298-07-7 -
![Bis(2-ethylhexyl) Hydrogen Phosphate [for Rare Metals Extraction] CAS 298-07-7](https://img.chemicalbook.in//Content/image/CP5.jpg) Bis(2-ethylhexyl) Hydrogen Phosphate [for Rare Metals Extraction] CAS 298-07-7View Details
Bis(2-ethylhexyl) Hydrogen Phosphate [for Rare Metals Extraction] CAS 298-07-7View Details
298-07-7 -
 Bis(2-ethylhexyl) phosphate, 97% CAS 298-07-7View Details
Bis(2-ethylhexyl) phosphate, 97% CAS 298-07-7View Details
298-07-7 -
 Di-2-Ethylhexyl Phosphoric AcidView Details
Di-2-Ethylhexyl Phosphoric AcidView Details
298-07-7
