BIS(2-CHLOROISOPROPYL)ETHER
Synonym(s):2-Chloroisopropyl ether;2-Chloroisopropyl ether solution
- CAS NO.:108-60-1
- Empirical Formula: C6H12Cl2O
- Molecular Weight: 171.06
- MDL number: MFCD00152274
- EINECS: 203-598-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
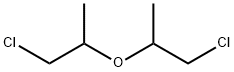
What is BIS(2-CHLOROISOPROPYL)ETHER?
Description
Bis-(2-chloroisopropyl) ether is a byproduct in the synthesis of ethylene and propylene glycol that has been found in municipal drinking water in the United States and waterways in the Netherlands where it is considered a persistent organic pollutant (POP). It is mutagenic in the S. typhimurium strains TA 1535 and TA 100 but lacks mutagenic activity in mice.
Chemical properties
Clear Colourless Oil
Chemical properties
Dichloroisopropyl ether is a colorless liquid.
Physical properties
Colorless to light brown oily liquid. Verschueren (1983) reported an odor threshold concentration of 320 ppb.
The Uses of BIS(2-CHLOROISOPROPYL)ETHER
Bis(2-chloroisopropyl)ether is used as a sol vent for resins, waxes, and oils, and inorganic synthesis.
The Uses of BIS(2-CHLOROISOPROPYL)ETHER
Bis(2-chloroisopropyl) Ether is an organochlorine pesticide. Bis(2-chloroisopropyl) Ether is used as a nematicide to control parasitic nematodes in agriculture.
The Uses of BIS(2-CHLOROISOPROPYL)ETHER
Apparently used as a nematocide in Japan but is not registered in the U.S. for use as a pesticide.
Definition
ChEBI: Bis(2-chloro-1-methylethyl)ether is an ether.
General Description
Colorless to light brown liquid. Odor threshold concentration 200 μg/L.
Air & Water Reactions
Subject to peroxidation in air. Insoluble in water.
Reactivity Profile
BIS(2-CHLOROISOPROPYL)ETHER oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick 1979 p.151-154, 164].
Health Hazard
Bis(2-chloroisopropyl)ether exhibited moderately toxic and carcinogenic actions intest animals. The acute inhalation toxicity of this compound is considerably lowerthan those of bis(chloromethyl)ether and bis(chloroethyl)ether. Exposure to its vapors cancause irritation of the eyes and upper respiratory tract. Inhalation of 700 ppm of this com pound in air for 5 hours proved fatal to rats
The oral toxicity of bis(2-chloroisopropyl)ether in rats was found to be moderate, withan LD50 value of 240 mg/kg.
The compound is carcinogenic to animals.Although there is no evidence of its carcino genicity in humans, exposure may cause lungcancer.
Fire Hazard
BIS(2-CHLOROISOPROPYL)ETHER is combustible.
Potential Exposure
BCIE was previously used as a solvent and as an extractant. It may be formed as a by-product of propylene oxide production. It has been found in industrial waste water and in natural water.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least20 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Source
No MCLGs or MCLs have been proposed, however, a DWEL of 1
mg/L was recommended (U.S. EPA, 2000).
A waste by-product in the manufacture of propylene glycol (quoted, Verschueren, 1983).
Environmental Fate
Biological. When bis(2-chloroisopropyl)ether (5 and 10 mg/L) was statically incubated
in the dark at 25°C with yeast extract and settled domestic wastewater inoculum, complete
biodegradation was achieved after 14 days (Tabak et al., 1981).
Chemical/Physical. Kollig (1993) reported that bis(2-chloroisopropyl)ether is subject
to hydrolysis forming hydrochloric acid and the intermediate (2-hydroxy-isopropyl-2-
chloroisopropyl)ether. The latter undergoes further hydrolysis yielding bis
storage
Color Code—Blue: Health Hazard/Poison:Store in a secure poison location. Ethers tend to formunstable peroxides. Before entering confined space whereBCIE may be present, check to make sure that an explosiveconcentration does not exist. Store in tightly closed containers in a cool, well-ventilated area away from oxidizingmaterials. Metal containers involving the transfer of thischemical should be grounded and bonded. Where possible,automatically pump liquid from drums or other storage containers to process containers. Drums must be equipped withself-closing valves, pressure vacuum bungs, and flamearresters. Use only nonsparking tools and equipment, especially when opening and closing containers of this chemical.Sources of ignition, such as smoking and open flames,are prohibited where this chemical is used, handled, orstored in a manner that could create a potential fire orexplosion hazard.
Shipping
UN2490 Dichloroisopropyl ether, Hazard Class: 6.1; Labels: 6.1—Poisonous materials.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. It may form dangerous peroxides upon standing; may explode when heated.
Waste Disposal
Use special incinerator due to high HCl content, such as seagoing incinerator ships.
Properties of BIS(2-CHLOROISOPROPYL)ETHER
| Melting point: | -97℃ |
| Boiling point: | 242.21°C (rough estimate) |
| Density | 1.1030 |
| vapor pressure | 0.56 (quoted, Kawamoto and Urano, 1989) |
| refractive index | 1.4505 (estimate) |
| Flash point: | 11 °C |
| storage temp. | 0-6°C |
| solubility | Soluble in acetone, ethanol, benzene, ether (Weast, 1986), and many other solvents including
methanol, propanol, and 2-butanone. |
| form | neat |
| Henry's Law Constant | 0.24 at 25 °C (static headspace-GC, Kawamoto and Urano, 1989) |
| Exposure limits | No exposure limit is set for this compound. Carcinogen — Animal Limited Evidence (IARC). |
| CAS DataBase Reference | 108-60-1 |
| IARC | 3 (Vol. 41, Sup 7, 71) 1999 |
| EPA Substance Registry System | Bis(2-chloro-1-methylethyl) ether (108-60-1) |
Safety information for BIS(2-CHLOROISOPROPYL)ETHER
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H332:Acute toxicity,inhalation |
Computed Descriptors for BIS(2-CHLOROISOPROPYL)ETHER
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 Bis-(2-chloroisopropyl) ether CAS 108-60-1View Details
Bis-(2-chloroisopropyl) ether CAS 108-60-1View Details
108-60-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8