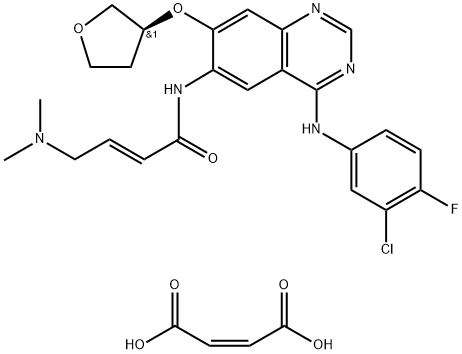
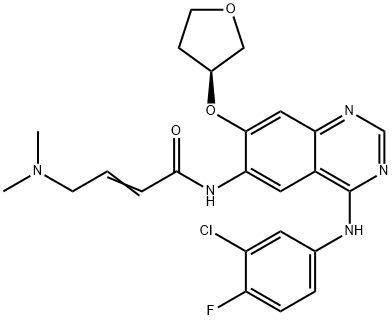
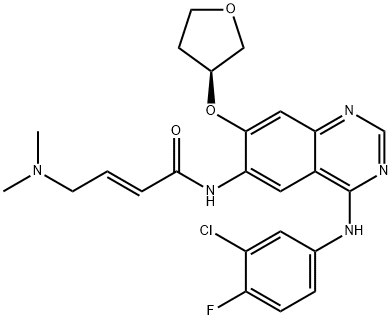
Afatinib dimaleate
- CAS NO.:850140-73-7
- Empirical Formula: C28H29ClFN5O7
- Molecular Weight: 602.02
- MDL number: MFCD28969755
- EINECS: 810-416-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
What is Afatinib dimaleate?
Description
Afatinib dimaleate was approved by the U.S. Food and Drug Administration (FDA) in 2013 for the treatment of non-small cell lung cancer (NSCLC). Specifically, it was approved for patients presenting with metastatic NSCLC tumors which contain epidermal growth factor receptor (EGFR) exon deletions or exon 21 mutations. Afatinib dimaleate is a covalent inhibitor of ErbB tyrosine kinases (tyk), which downregulates ErbB signaling by irreversible binding of EGFR tyk binding sites. While no manufacturing route has been disclosed to date, the most scalable published route likely derives from two Boehringer Ingelheim patents.
The Uses of Afatinib dimaleate
Afatinib Dimaleate is a salt of Afatinib {BIBW 2992), an aminocrotonylamino-substituted quinazoline derivative used for treating cancer and diseases of the respiratory tract, lungs, gastrointestinal tract, bile duct, and gallbladder. An anilino-quinazoline that irreversibly inhibits EGFR and HER2 kinase activity.
Definition
ChEBI: Afatinib dimaleate is a maleate salt obtained by combining afatinib with two molar equivalents of maleic acid. Used for the first-line treatment of patients with metastatic non-small cell lung cancer. It has a role as a tyrosine kinase inhibitor and an antineoplastic agent. It contains an afatinib.
General Description
Afatinib dimaleate is the dimaleate form of afatinib. It is a tyrosine kinase inhibitor of epidermal growth factor receptors (ERBB RECEPTORS) and an anti-angiogenic agent. Afatinib dimaleate has anti-tumour activity and is mainly used for the treatment of patients with metastatic non-small cell lung cancer (which has metastasised or spread to other parts of the body), or certain tumours with mutations in the EGFR gene and patients whose disease has worsened after platinum-based chemotherapy. It is also used for the treatment of esophageal squamous cell carcinoma (ESCC) and gastric cancer.
Biological Activity
Afatinib dimaleate is an orally bioavailable and irreversible dual-specific inhibitor of the ErbB family (EGFR and HER2). IC50 are 0.4, 0.5, 10, 14, 1 nM for EGFRL858R, EGFRwt, EGFR L858R/T790M, ErbB2 (HER2) and ErbB4 (HER4), respectively. Afatinib dimaleate is available for cancer (esophageal squamous cell carcinoma (ESCC), non-small cell lung cancer (NSCLC) and gastric cancer) studies.
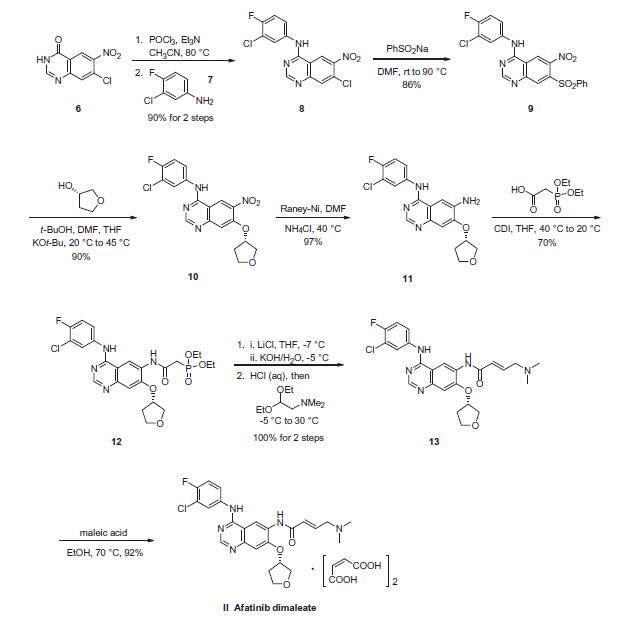
Synthesis
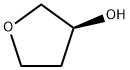
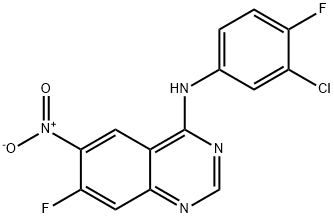
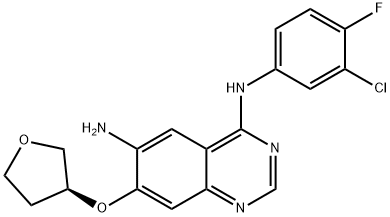
Nitroquinazolinone (6), which is commercially available, was first chlorinated with phosphorous oxychloride (POCl3) followed by treatment with commercial 3-chloro-4-fluoroaniline (7) to afford SNAr adduct 8 in 90% yield over two steps. Sulfonylation to afford 9 (86%) and subsequent displacement with (S)-tetrahydrofuran- 3-ol gave 10 in 90% yield. Raney¨CNickel reduction of the nitro group delivered 11 in 97% yield, which set the stage for the final side-chain functionalization. 2-(Diethoxyphosphoryl) acetic acid and N,N0-carbonyldiimidazole (CDI) were pre-mixed and added to aniline 11 to afford 12 in 70% isolated yield. Next, a Horner¨CWadsworth¨CEmmons homologation gave the (E)-olefin 13 in quantitative yield, followed by maleate salt formation (92%) to deliver the final API. The final five steps of this synthesis have been successfully demonstrated on multi-kilogram scale.
storage
Store at -20°C
Properties of Afatinib dimaleate
| Melting point: | >237oC (dec.) |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Pale Yellow |
Safety information for Afatinib dimaleate
Computed Descriptors for Afatinib dimaleate
| InChIKey | LIENDGDDWJRJLO-LBXKZPGENA-N |
| SMILES | C(/C(=O)O)=C/C(=O)O.N(C1C=CC(F)=C(Cl)C=1)C1=NC=NC2C=C(O[C@@H]3COCC3)C(NC(=O)/C=C/CN(C)C)=CC1=2 |&1:25,r| |
Afatinib dimaleate manufacturer
Shilpa Medicare Limited (SML)
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![4-QuinazolinaMine, N-(3-chloro-4-fluorophenyl)-6-nitro-7-[[(3S)-tetrahydro-3-furanyl]oxy]-](https://img.chemicalbook.in/CAS/GIF/314771-88-5.gif)

You may like
-
 850140-73-7 Afatinib dimaleate 99%View Details
850140-73-7 Afatinib dimaleate 99%View Details
850140-73-7 -
 850140-73-7 98%View Details
850140-73-7 98%View Details
850140-73-7 -
 Afatinib dimaleate 95% CAS 850140-73-7View Details
Afatinib dimaleate 95% CAS 850140-73-7View Details
850140-73-7 -
 Afatinib dimaleate CAS 850140-73-7View Details
Afatinib dimaleate CAS 850140-73-7View Details
850140-73-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1