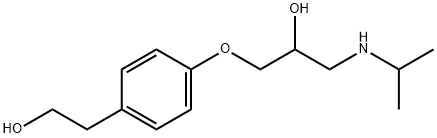
Betaxolol
- CAS NO.:63659-18-7
- Empirical Formula: C18H29NO3
- Molecular Weight: 307.43
- MDL number: MFCD00242958
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-18 11:31:11
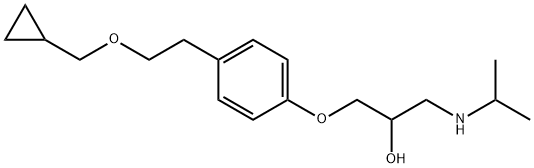
What is Betaxolol?
Absorption
Absorption of an oral dose is complete. There is a small and consistent first-pass effect resulting in an absolute bioavailability of 89% ± 5% that is unaffected by the concomitant ingestion of food or alcohol.
Toxicity
Oral LD50s are 350 to 400 mg betaxolol/kg in mice and 860 to 980 mg/kg in rats. Predicted symptoms of overdose include bradycardia, congestive heart failure, hypotension, bronchospasm, and hypoglycemia.
Chemical properties
White Crystalline Solid
Originator
Kerlone,Carriere,France,1983
The Uses of Betaxolol
Cardioselective 1-adrenergic blocker. An antihypertensive; antiglaucoma
The Uses of Betaxolol
Cardioselective β1-adrenergic blocker. An antihypertensive; antiglaucoma.
Background
A cardioselective beta-1-adrenergic antagonist with no partial agonist activity.
What are the applications of Application
Betaxolol is Betaxolol is a cardioselective β1-adrenergic blocker, as well as an antihypertensive and an antiglaucoma.
Indications
For the management of hypertension.
Definition
ChEBI: A propanolamine that is 3-aminopropane-1,2-diol in which the hydrogen of the primary hydoxy is substituted by a 4-[2-(cyclopropylmethoxy)ethyl]phenyl group and one of the hydrogens attached to the amino group is substituted by isopropyl. It is a selective beta1-receptor blocker and is used in the treatment of glaucoma as well as hypertension, arrhythmias, and coronary heart disease. It is also used to reduce non-fatal cardiac events in patients with heart failure.
Manufacturing Process
(1) 1 g of sodium hydroxide pellets (0.025 mol) is added to a suspension of
3.8 g of 4-[2-(cyclopropylmethoxy)-ethyl]-phenol in 30 ml of water. When the
solution becomes homogenous, 2.3 ml of epichlorohydrin are added and the
mixture is stirred for 8 hours. It is then extracted with ether and the extract is
washed with water, dried over sodium sulfate and evaporated to dryness. The
compound is purified by passing it over a silica column. 2.4 g of 1-[4-[2-
(cyclopropylmethoxy)ethyl]-phenoxy]-2,3-epoxy-propane are thus obtained.
(2) 4.9 g of the preceding compound (0.02 mol) are condensed with 25 ml of
isopropylamine by contact for 8 hours at ambient temperature and then by
heating for 48 hours at the reflux temperature. After evaporation to dryness,
the compound obtained is crystallized from petroleum ether. 5 g (yield 80%)
of 2-[[4-(2-cyclopropylmethoxy)-ethyl]-phenoxy]-3-isopropylaminopropan-2-ol
are thus obtained, melting point 70° to 72°C.
The hydrochloride is prepared by dissolving the base in the minimum amount
of acetone and adding a solution of hydrochloric acid in ether until the pH is
acid. The hydrochloride which has precipitated is filtered off and is
recrystallized twice from acetone, melting point 116°C.
brand name
Betoptic (Alcon); Kerlone (Sanofi Aventis).
Therapeutic Function
Beta-adrenergic blocker
Biological Activity
betaxolol is a cardioselective beta-adrenergic receptor blocking agent. betaxolol (5 mg/kg via i.p. injection) was administered at 24 and then 44 h following the final chronic cocaine administration. animals treated with betaxolol during cocaine withdrawal
Pharmacokinetics
Betaxolol is a competitive, beta(1)-selective (cardioselective) adrenergic antagonist. Betaxolol is used to treat hypertension, arrhythmias, coronary heart disease, glaucoma, and is also used to reduce non-fatal cardiac events in patients with heart failure. Activation of beta(1)-receptors (located mainly in the heart) by epinephrine increases the heart rate and the blood pressure, and the heart consumes more oxygen. Drugs such as betaxolol that block these receptors therefore have the reverse effect: they lower the heart rate and blood pressure and hence are used in conditions when the heart itself is deprived of oxygen. They are routinely prescribed in patients with ischemic heart disease. In addition, beta(1)-selective blockers prevent the release of renin, which is a hormone produced by the kidneys which leads to constriction of blood vessels. Betaxolol is lipophilic and exhibits no intrinsic sympathomimetic activity (ISA) or membrane stabilizing activity.
Veterinary Drugs and Treatments
Betaxolol HCl is a specific Beta1 adrenergic blocking agent which reduces aqueous humor production by decreasing cyclic-AMP synthesis in the ciliary body. This drug is a suitable substitute for timolol and because of its specific Beta1 activity, might be a first choice Beta blocking agent for patients with concurrent respiratory disease. Either levobunolol HCl or betaxolol HCl would be the first choice Beta blocking agent in a feline patient with glaucoma and asthma, although a topical carbonic anhydrates inhibitor should be considered before a Beta blocking agent in this situation. Betaxolol and the other Beta blockers should be used with caution in patients with cardiac disease.
Metabolism
Primarily hepatic. Approximately 15% of the dose administered is excreted as unchanged drug, the remainder being metabolites whose contribution to the clinical effect is negligible.
Metabolism
Absorption of an oral dose of betaxolol (Kerlone, Betoptic) is almost complete. The drug is subject to a slight first-pass effect such that the absolute bioavailability of the drug is about 90%.Approximately 50% of administered betaxolol binds to plasma proteins, and its plasma half-life is about 20 hours; it is suitable for dosing once per day.The primary route of elimination is by liver metabolism, with only 15% of unchanged drug being excreted.
Properties of Betaxolol
| Melting point: | 61-63°C |
| Boiling point: | 448.0±40.0 °C(Predicted) |
| Density | 1.067±0.06 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | pKa 9.21 (Uncertain) |
| color | White |
| CAS DataBase Reference | 63659-18-7(CAS DataBase Reference) |
Safety information for Betaxolol
Computed Descriptors for Betaxolol
Betaxolol manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Betaxolol 98%View Details
Betaxolol 98%View Details -
 Betaxolol 98% (HPLC) CAS 63659-18-7View Details
Betaxolol 98% (HPLC) CAS 63659-18-7View Details
63659-18-7 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
