BETA-HCH
Synonym(s):β-1,2,3,4,5,6-Hexachlorocyclohexane
- CAS NO.:319-85-7
- Empirical Formula: C6H6Cl6
- Molecular Weight: 290.83
- MDL number: MFCD00135948
- EINECS: 206-271-3
- Update Date: 2024-12-18 14:08:57
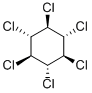
What is BETA-HCH?
Chemical properties
BHC is a white-to-brownish crystalline solid with a musty, phosgene-like odor.
Physical properties
Although β-BHC is a solid at room temperature, the odor threshold concentration is 0.32 μg/kg (Sigworth, 1964).
The Uses of BETA-HCH
β-1,2,3,4,5,6-Hexachlorocyclohexane is an organochloride which is one of the isomers of hexachlorocyclohexane and is also an byproduct of insecticide Lindane (L465990).
The Uses of BETA-HCH
Insecticide.
Definition
ChEBI: The beta-isomer of hexachlorocyclohexane.
General Description
Colorless crystals. Corrosive to aluminum and many other metals.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
Halogenated aliphatic compounds, such as BETA-HCH, are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Materials in this group may be incompatible with strong oxidizing and reducing agents. Also, they may be incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. Dehydrochlorination occurs in the presence of alkalis.
Health Hazard
ACUTE/CHRONIC HAZARDS: Highly toxic carcinogen. May cause irritation on contact. Hazardous decomposition products.
Safety Profile
Confirmed carcinogen with experimental neoplastigenic data. Mildly toxic by ingestion. When heated to decomposition it emits very toxic fumes of Cl-, HCl, and phosgene. See also BENZENE HEXACHLORIDE and other benzenehexachloride entries.
Potential Exposure
The major commercial usage of BHC is based upon its insecticidal properties. α-BCH is used as an Agricultural chemical, pesticide, pharmaceutical, and veterinary drug. The 7-isomer has the highest acute toxic ity, but the other isomers are not without activity. It is gen erally advantageous to purify the 7-isomer from the less active isomers. The γ-isomer acts on the nervous system of insects, principally at the level of the nerve ganglia. As a result, lindane has been used against insects in a wide range of applications including treatment of animals, buildings, humans for ectoparasites, clothes; water for mosquitoes; living plants; seeds and soils. Some applications have been abandoned due to excessive residues, e.g., stored food stuffs. By voluntary action, the principal domestic producer of technical grade BHC requested cancellation of its BHC registrations on September 1, 1976. As of July 21, 1978, all registrants of pesticide products containing BHC voluntar ily canceled their registrations or switched their former BHC products to lindane formulations.
Environmental Fate
Soil. No biodegradation of β-BHC was observed under denitrifying and sulfate-reduc ing conditions in a contaminated soil collected from The Netherlands (Bachmann et al.,
1988). In four successive 7-day incubation periods, β-BHC (5 and 10 mg/L) was recalci trant to degradation in a settled domestic wastewater inoculum (Tabak et al., 1981).
Chemical/Physical. Emits very toxic fumes of chlorides, hydrochloric acid and phos gene when heated to decomposition (Lewis, 1990). β-BHC will not hydrolyze to any
reasonable extent (Kollig, 1993).
Shipping
UN2761 Organochlorine pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explo sions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Decomposes on contact with powdered iron, aluminum, zinc, and on contact with strong bases producing trichlorobenzene.
Waste Disposal
A process has been developed for the destructive pyrolysis of benzene hexachloride @ 400 500℃ with a catalyst mixture which contains 5 10% of either cupric chloride, ferric chloride; zinc chloride; or aluminum chloride on activated carbon.
Properties of BETA-HCH
| Melting point: | ≥300 °C |
| Boiling point: | 373.64°C (rough estimate) |
| Density | 1.9 g/cm3 |
| vapor pressure | 4.66 at 25 °C (Banerjee et al., 1990) |
| refractive index | 1.630 (589.3 nm) |
| Flash point: | 11 °C |
| storage temp. | APPROX 4°C
|
| solubility | Soluble in ethanol, benzene, and chloroform (Weast, 1986) |
| form | Solid |
| Water Solubility | 0.24mg/L(25 ºC) |
| BRN | 1907338 |
| Henry's Law Constant | 0.53 at 5 °C, 0.91 at 10 °C, 2.17 at 20 °C, 5.23 at 30 °C, 8.68 at 35 °C:in 3% NaCl solution: 1.09
at 5 °C, 1.48 at 15 °C, 2.76 at 25 °C, 4.24 at 35 °C (dynamic headspace-GC, Sahsuvar et al.,
2003 |
| EPA Substance Registry System | .beta.-Hexachlorocyclohexane (319-85-7) |
Safety information for BETA-HCH
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H312:Acute toxicity,dermal H351:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for BETA-HCH
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran






You may like
-
 B-BHC 1000UG/ML IN ACETONE CAS 319-85-7View Details
B-BHC 1000UG/ML IN ACETONE CAS 319-85-7View Details
319-85-7 -
 β-HCH CAS 319-85-7View Details
β-HCH CAS 319-85-7View Details
319-85-7 -
 β-HCH CAS 319-85-7View Details
β-HCH CAS 319-85-7View Details
319-85-7 -
 β-BHC CASView Details
β-BHC CASView Details -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1