Besifloxacin hydrochloride
Synonym(s):7-[-3-Aminoazepam-1-yl]-8-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid hydrochloride
- CAS NO.:405165-61-9
- Empirical Formula: C19H22Cl2FN3O3
- Molecular Weight: 430.3
- MDL number: MFCD14636632
- EINECS: 696-612-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
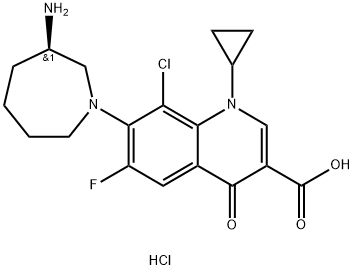
What is Besifloxacin hydrochloride?
Description
Besifloxacin is a new fluoroquinolone antibacterial for ophthalmic use. It is indicated for the treatment of bacterial conjunctivitis, one of the most common ocular infections encountered in the primary care setting. Although the previously marketed fluoroquinolones ciprofloxacin, levofloxacin, ofloxacin, gatifloxacin, and moxifloxacin have been widely used to treat bacterial conjunctivitis, their continued utility is hampered by the emergence of resistance among key ocular isolates such as Staphylococcus aureus. Besifloxacin is the first fluoroquinolone developed exclusively for topical ophthalmic use. Unlike the previously marketed fluoroquinolones, besifloxacin has not been used systemically and is not in development as a systemic agent. Its exclusive indication as a topical agent is expected to reduce the overall environmental exposure of bacteria to besifloxacin, which may contribute to a lower risk for the emergence of bacterial resistance. Fluoroquinolones derive their antibacterial activity via inhibition of two essential bacterial enzymes, DNA gyrase and topoisomerase IV, which regulate processes of DNA replication. Besifloxacin inhibits DNA gyrase and topoisomerase IV from Streptococcus pneumoniae (IC50 = 1 and 0.4 mg/ L, respectively) and Escherichia coli (IC50 = 1 and 10 mg/L, respectively).
Chemical properties
Pale Yellow Solid
Originator
SSP Co. Ltd. (Japan) (Japan)
The Uses of Besifloxacin hydrochloride
Besifloxacin HCl is a fourth-generation fluoroquinolone antibiotic
The Uses of Besifloxacin hydrochloride
A Fluoroquinolone antibiotic.
What are the applications of Application
Besifloxacin Hydrochloride is a Fluoroquinolone antibiotic
brand name
Besivance
Side Effects
The most common adverse event reported in patients treated with besifloxacin ophthalmic suspension was conjunctival redness. Other less common adverse events were blurred vision, eye pain, eye irritation, eye pruritus, and headache.
Synthesis
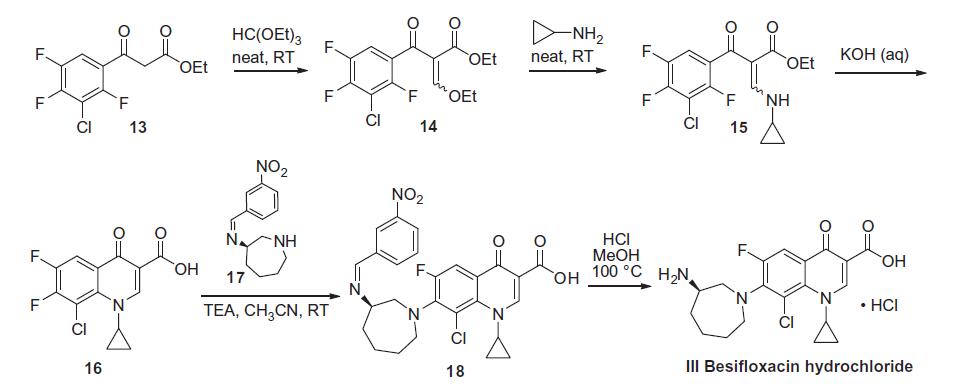
Besifloxacin is a fourth-generation fluoroquinolone antibiotic which is marketed as besifloxacin hydrochloride. It was originally developed by the Japanese firm SSP Co. Ltd and designated SS734. SSP then licensed U.S. and European rights of SS734 for ophthalmic use to InSite Vision, Inc., in 2000, who then developed an eye drop formulation (ISV-403) and conducted preliminary clinical trials before selling the product and all rights to Bausch & Lomb in 2003. The eye drop was approved by the United States Food and Drug Administration (FDA) on May 29, 2009 and marketed under the trade name Besivance. Besifloxacin has been found to inhibit production of pro-inflammatory cytokines in vitro. The synthesis of besifloxacin commences with commercially available ethyl 3-(3-chloro-2,4,5-trifluorophenyl)-3-oxopropanoate.Condensation of this ketoester with triethyl orthoformate resulted in a mixture of vinylogous esters 14. Substitution with cyclopropanamine converts 14 to the vinylogous amide 15 as an unreported distribution of cis- and trans-isomers. This mixture was treated with base at elevated temperature to give 16. Presumably, the trans-isomer isomerizes to the cis-isomer, which subsequently undergoes an intramolecular nucleophilic aromatic substitution with concomitant saponification to construct quinolone acid 16. Quinolone 16 is then subjected to another nucleophilic substitution involving readily available iminoazepine 17 and the displacement reaction proceeds regioselectively to furnish the atomic framework of besifloxacin (18). Acidic methanolysis of 18 at elevated temperature gave besiflozacin (III).
Properties of Besifloxacin hydrochloride
| Melting point: | >210°C (dec.) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Methanol (Slightly, Heated, Sonicated), Water (Slightly, Heated, Sonicated) |
| form | Solid |
| color | Off-White to Light Beige |
Safety information for Besifloxacin hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Besifloxacin hydrochloride
Besifloxacin hydrochloride manufacturer
Archerchem Healthcare Pvt., Ltd. (part of Archerchem Group)
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 405165-61-9 BESIFLOXACIN HCL 98%View Details
405165-61-9 BESIFLOXACIN HCL 98%View Details
405165-61-9 -
 405165-61-9 98%View Details
405165-61-9 98%View Details
405165-61-9 -
 Besifloxacin hydrochloride 98%View Details
Besifloxacin hydrochloride 98%View Details
405165-61-9 -
 Besifloxacin hydrochloride 405165-61-9 98%View Details
Besifloxacin hydrochloride 405165-61-9 98%View Details
405165-61-9 -
 405165-61-9 98%View Details
405165-61-9 98%View Details
405165-61-9 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
