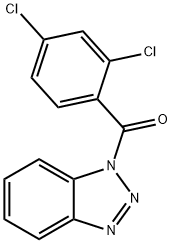
BENZOTRIAZOL-1-YL-(2,4-DICHLORO-PHENYL)-METHANONE
- CAS NO.:200626-61-5
- Empirical Formula: C13H7Cl2N3O
- Molecular Weight: 292.12
- MDL number: MFCD00195103
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
What is BENZOTRIAZOL-1-YL-(2,4-DICHLORO-PHENYL)-METHANONE?
The Uses of BENZOTRIAZOL-1-YL-(2,4-DICHLORO-PHENYL)-METHANONE
Histone deacetylase (HDAC) inhibitors?are able to interrupt cell cycle progression in transformed cell lines and may be explored as new clinical agents in cancer therapy. 1-(2,4-Dichlorobenzoyl)-1H-benzotriazole has been shown to suppress the biological effects induced by the HDAC inhibitor, Trichostatin A (T774710).
What are the applications of Application
ITSA1 is an HDAC activator via TSA suppression
Definition
ChEBI: 1-(2,4-dichlorobenzoyl)-1H-1,2,3-benzotriazole is a member of the class of benzamides that is obtained by the formal condensation of 2,4-dichlorobenzoic acid and benzotriazole. It acts as an inhibitor for tubulin acetylation mediated by trichostatin A. It has a role as an inhibitor. It is a member of benzotriazoles, a member of benzamides and a dichlorobenzene. It is functionally related to a 2,4-dichlorobenzoic acid and a benzotriazole.
Biological Activity
itsa-1 (itsa1) is an hdac activator through trichostatin a (tsa) suppression.trichostatin a (tsa), a streptomyces metabolite, can specifically inhibit mammalian histone deacetylase at a nanomolar concentration causing accumulation of highly acetylated histone molecules in mammalian cells.
in vitro
previous study reported that in murine embryonic stem cells, tsa treatment for 23 hours inhibited brdu incorporation compared with the control. tsa-pretreated cells incubated with itsa1, however, could incorporate brdu at concentrations where it was inhibited by tsa alone. moreover, itsa1 treatment was able to revert the tsa-arrested population to a normal cell cycle distribution. tsa treatment at 300 nm to a549 cells for 2 hours noticeably increased the levels of acetyl-histone h3, whereas subsequent incubation with itsa1 at 50 μm for 2 hours reduced histone acetylation to the baseline level. in addition, cells pretreated with itsa1 before addition of tsa showed increased acetylation levels, which was a characteristic of tsa treatment alone. these results suggested that the target of itsa1 was not present until induced by tsa. furthermore, the itsa1 treatment alone at 50 or 100 μm was not effective on hdac activity, demonstrating that itsa1 could directly affect hdac function [1].
References
[1] koeller km, haggarty sj, perkins bd, leykin i, wong jc, kao mc, schreiber sl. chemical genetic modifier screens: small molecule trichostatin suppressors as probes of intracellular histone and tubulin acetylation. chem biol. 2003 may;10(5):397-410.
Properties of BENZOTRIAZOL-1-YL-(2,4-DICHLORO-PHENYL)-METHANONE
| Melting point: | 119-121°C |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | DMSO: ~28mg/mL |
| form | solid |
| color | White to Off-White |
Safety information for BENZOTRIAZOL-1-YL-(2,4-DICHLORO-PHENYL)-METHANONE
Computed Descriptors for BENZOTRIAZOL-1-YL-(2,4-DICHLORO-PHENYL)-METHANONE
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 6-Bromo-3-iodo-1-methyl-1H-indazole N-Boc-L-proline methyl ester 2-(BOC-Amino)4-picoline 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazoleRelated products of tetrahydrofuran
You may like
-
 55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 -
 181228-33-1 99%View Details
181228-33-1 99%View Details
181228-33-1 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 Chloro Uracil 99%View Details
Chloro Uracil 99%View Details
1820-81-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5