65-85-0
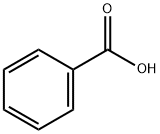
Product Name:
Benzoic acid
Formula:
C7H6O2
Synonyms:
Acidum benzoicum;Benzoic acid;Benzoic acid solution;Phenylformic acid, Benzene carboxylic acid
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Benzoic acid appears as a white crystalline solid. Slightly soluble in water. The primary hazard is the potential for environmental damage if released. Immediate steps should be taken to limit spread to the environment. Used to make other chemicals, as a food preservative, and for other uses. |
|---|---|
| Color/Form | Monoclinic tablets, plates, leaflets |
| Odor | Odorless or with a slight benzaldehyde odor |
| Taste | ALMOST TASTELESS /BENZOIC ACID USP/ |
| Boiling Point | 480 °F at 760 mmHg (NTP, 1992) |
| Melting Point | 252.3 °F (NTP, 1992) |
| Flash Point | 250 °F (NTP, 1992) |
| Solubility | less than 1 mg/mL at 68 °F (NTP, 1992) |
| Density | 1.316 at 82.4 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Density | 4.21 (NTP, 1992) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 1 mmHg at 205 °F (NTP, 1992) |
| LogP | 1.87 |
| LogS | -1.55 |
| Stability/Shelf Life | A 0.1% w/v aqueous solution of benzoic acid has been reported to be stable for at least 8 weeks when stored in polyvinyl chloride bottels, at room temperature. |
| Autoignition Temperature | 1063 °F (USCG, 1999) |
| Decomposition | When heated to decomp it emits acrid smoke and irritating fumes. |
| Viscosity | 1.26 cP at 130 °C |
| Heat of Combustion | 3227 kJ/mole |
| Heat of Vaporization | 534 KJ/mol at 140 °C, 425 Kj/mol at 249 °C |
| pH | About 4 (solution in water) |
| Surface Tension | 31 mN/m at 130 °C |
| Ionization Efficiency | Negative |
| Refractive Index | Index of refraction = 1.504 at 132 °C/D |
| Dissociation Constants | 4.207 |
| Collision Cross Section | 121.84 Ų [M-H]- [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)] |
| Kovats Retention Index | 1160 1174 1191 1168 1159 1160 1165 1131 1131 1164 1148 1200 1214 1161 1152 1138 1197 1155 1210 1135 1167 1170 1143 1163 1149 1134.5 1180 196.52 200.65 202.69 |
| Other Experimental Properties | Partially sublimes at 100 °C |
| Chemical Classes | Other Classes -> Benzoic Acid Derivatives |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
COMPUTED DESCRIPTORS
| Molecular Weight | 122.12 g/mol |
|---|---|
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 122.036779430 g/mol |
| Monoisotopic Mass | 122.036779430 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 104 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Benzoic acid appears as a white crystalline solid. Slightly soluble in water. The primary hazard is the potential for environmental damage if released. Immediate steps should be taken to limit spread to the environment. Used to make other chemicals, as a food preservative, and for other uses.
