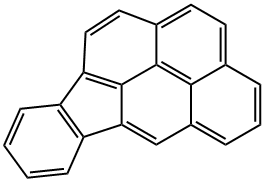
BENZO(B)FLUORANTHENE
Synonym(s):REF;THOC4;2,3-Benzfluoranthene;3,4-Benz[e]acephenanthrylene;3,4-Benzfluoranthene
- CAS NO.:205-99-2
- Empirical Formula: C20H12
- Molecular Weight: 252.31
- MDL number: MFCD00010582
- EINECS: 205-911-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
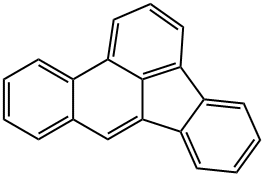
What is BENZO(B)FLUORANTHENE?
Description
: Benzo(b)fluoranthene is a colorless, needleshaped solid. Molecular weight= 252.32; Boilingpoint = 480℃; Freezing/Melting point= 168℃; Vaporpressure = 5.0 3 10 2 7 mmHg. Hazard Identification (basedon NFPA-704 M Rating System): Health 3, Flammability 1,Reactivity 0. Practically insoluble in water; solubility =1 mg/mL at 19℃.
Chemical properties
off-white to tan powder
Chemical properties
Benzo(b)fluoranthene is a colorless, needleshaped solid.
Physical properties
Colorless to pale yellow to yellow-orange needles or crystals from benzene, toluene, ethylbenzene or acetic acid.
The Uses of BENZO(B)FLUORANTHENE
Benz[e]acephenanthrylene is a polycyclic aromatic hydrocarbon that was detected in significant levels in airborne polluants, auto exhaust and tobacco and marijuana smoke.
Definition
ChEBI: An ortho- and peri-fused polycyclic arene that consists of a benzene ring fused with a acephenanthrylene ring.
Synthesis Reference(s)
Tetrahedron Letters, 36, p. 2403, 1995 DOI: 10.1016/0040-4039(95)00314-3
General Description
Needles or yellow fluffy powder.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
BENZO(B)FLUORANTHENE can react with strong oxidizing agents. May react with electrophiles, peroxides, nitrogen oxides and sulfur oxides
Hazard
Confirmed carcinogen.
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition, BENZO(B)FLUORANTHENE emits acrid smoke and irritating fumes.
Health Hazard
Acute oral toxicity data are not available.There is sufficient evidence on the carcinogenicity of this compound in animals. Itproduced tumors at the site of application.Cancers in lungs and skin have been observedin animals.
Fire Hazard
Flash point data for BENZO(B)FLUORANTHENE are not available; however, BENZO(B)FLUORANTHENE is probably combustible.
Safety Profile
Confirmed carcinogen with experimental carcinogenic and tumorigenic data. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.
Potential Exposure
There is no commercial production of this compound. Benzo(b)fluoranthene is a chemical substance formed during the incomplete burning of fossil fuel, garbage, in cigarette smoke, or any organic matter and is Benzofluoranthene 399 found in smoke in general; it is carried into the air, where it condenses onto dust particles and is distributed into water and soil and on crops. B(b)F is a PAH and a component of coal tar pitch used in industry as a binder for electrodes. It is also a component of creosote, which is used to preserve wood. PAHs are also found in limited a mounts in bituminous materials and asphalt used for paving, roofing, and insulation. B(b)F has some use as a research chemical. It is available from some specialty chemical firms in low quantities (25 100 mg).
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, rinsemouth and get medical attention.
Carcinogenicity
Benzo[b]fluoranthenewas tested for carcinogenicity by dermal application in mice in multiple studies, intraperitoneal injection into mice in multiple studies, and intrapulmonary implantation into rats in one study. In all of these studies, benzo[b]-fluoranthene exhibited a significant carcinogenic activity.
Source
Benzo[b]fluoranthene and benzo[k]fluoranthene were detected in 8 diesel fuels at
concentrations ranging from 0.0027 to 3.1 mg/L with a mean value of 0.266 mg/L (Westerholm
and Li, 1994). Also present in low octane gasoline (0.16–0.49 mg/kg), high octane gasoline (0.26–
1.34 mg/kg), used motor oil (2.8–141.0 mg/kg), and bitumen (40 to 1,600 ppb), cigarette smoke (3
g/1,000 cigarettes), and gasoline exhaust (19 to 48 g/L) (quoted, Verschueren, 1983). Also
detected in asphalt fumes at an average concentration of 22.04 ng/m3 (Wang et al., 2001).
Nine commercially available creosote samples contained benzo[b]fluoranthene at concentrations
ranging from 2 to 96 mg/kg (Kohler et al., 2000).
Schauer et al. (2001) measured organic compound emission rates for volatile organic
compounds, gas-phase semi-volatile organic compounds, and particle phase organic compounds
from the residential (fireplace) combustion of pine, oak, and eucalyptus. The particle-phase
emission rates of benzo[b]fluoranthene were 0.790 mg/kg of pine burned, 0.400 mg/kg of oak
burned, and 0.327 mg/kg of eucalyptus burned.
Particle-phase tailpipe emission rate from a noncatalyst-equipped gasoline-powered automobile
was 37.3 μg/km (Schauer et al., 2002).
Environmental Fate
Biological. Ye et al. (1996) investigated the ability of Sphingomonas paucimobilis strain U.S.
EPA 505 (a soil bacterium capable of using fluoranthene as a sole source of carbon and energy) to
degrade 4, 5, and 6-ringed aromatic hydrocarbons (10 ppm). After 16 h of incubation using a
resting cell suspension, only 12.5% of benzo[b]fluoranthene had degraded. It was suggested that
degradation occurred via ring cleavage resulting in the formation of polar metabolites and carbon
dioxide.
Soil. The reported half-lives for benzo[b]fluoranthene in a Kidman sandy loam and McLaurin
sandy loam are 294 and 211 d, respectively (Park et al., 1990).
Photolytic. The atmospheric half-life was estimated to range from 1.43 to 14.3 h (Atkinson,
1987).
Chemical/Physical. Benzo[b]fluoranthene will not hydrolyze because it has no hydrolyzable
functional group (Kollig, 1993).
Storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Store in a cool, dry place. Aregulated, marked area should be established where thischemical is handled, used, or stored in compliance withOSHA Standard 1910.1045.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1—Poisonous materials, Technical Name Required.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
Residues and sorbent media may be packaged in 17H epoxy-lined drums and disposed of at an EPA-approved site. Destroy by permanganate oxidation, high-temperature incineration with scrubbing equipment, or microwave plasma treatment, if available. Confirm disposal procedures with responsible environmental engineer and regulatory officials.
Properties of BENZO(B)FLUORANTHENE
| Melting point: | 163-165 °C(lit.) |
| Boiling point: | 481°C(lit.) |
| Density | 1.1549 (estimate) |
| vapor pressure | 5 x 10-7 mmHg at 20 °C (U.S. EPA, 1982) |
| refractive index | 1.8390 (estimate) |
| Flash point: | -18 °C |
| storage temp. | 2-8°C |
| solubility | Soluble in most solvents (U.S. EPA, 1985) |
| form | neat |
| pka | >15 (Christensen et al., 1975) |
| color | White to Yellow to Green |
| Water Solubility | 1.2 μg/L at 25 °C (U.S. EPA, 1980a) |
| BRN | 1872553 |
| Henry's Law Constant | 2.47, 5.03, 11.74, 14.90, 20.53, and 36.52 at 10.0, 20.0, 35.0, 40.1, 45.0, and 55.0 °C, respectively
(wetted-wall column, ten Hulscher et al., 1992) |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 205-99-2(CAS DataBase Reference) |
| IARC | 2B (Vol. 92) 2010 |
| EPA Substance Registry System | Benzo[b]fluoranthene (205-99-2) |
Safety information for BENZO(B)FLUORANTHENE
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H350:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P391:Collect spillage. Hazardous to the aquatic environment P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. |
Computed Descriptors for BENZO(B)FLUORANTHENE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



![NAPHTHO[2,3-B]FLUORANTHENE](https://img.chemicalbook.in/CAS/GIF/206-06-4.gif)
You may like
-
 205-99-2 98%View Details
205-99-2 98%View Details
205-99-2 -
 205-99-2 Benzo(b)fluoranthene 98%+View Details
205-99-2 Benzo(b)fluoranthene 98%+View Details
205-99-2 -
![Benzo[b]fluoranthene CAS 205-99-2](https://img.chemicalbook.in//Content/image/CP5.jpg) Benzo[b]fluoranthene CAS 205-99-2View Details
Benzo[b]fluoranthene CAS 205-99-2View Details
205-99-2 -
![Benzo[b]fluoranthene CAS 205-99-2](https://img.chemicalbook.in//Content/image/CP5.jpg) Benzo[b]fluoranthene CAS 205-99-2View Details
Benzo[b]fluoranthene CAS 205-99-2View Details
205-99-2 -
![Benzo[b]fluoranthene CAS 205-99-2](https://img.chemicalbook.in//Content/image/CP5.jpg) Benzo[b]fluoranthene CAS 205-99-2View Details
Benzo[b]fluoranthene CAS 205-99-2View Details
205-99-2 -
 Anti-ALYREF antibody produced in rabbit CASView Details
Anti-ALYREF antibody produced in rabbit CASView Details -
![Benzo[b]fluoranthene CAS 205-99-2](https://img.chemicalbook.in//Content/image/CP5.jpg) Benzo[b]fluoranthene CAS 205-99-2View Details
Benzo[b]fluoranthene CAS 205-99-2View Details
205-99-2 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
