Bendazac
Synonym(s):[(1-Benzyl-1H-indazol-3-yl)oxy]acetic acid;Bendazolic acid
- CAS NO.:20187-55-7
- Empirical Formula: C16H14N2O3
- Molecular Weight: 282.29
- MDL number: MFCD00866158
- EINECS: 243-569-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
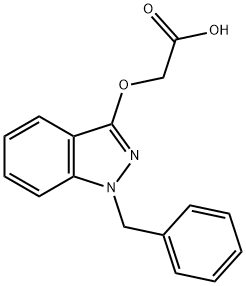
What is Bendazac?
Absorption
Administered as its lysine salt, a 500 mg oral tablet of bendazac is well absorbed into the human body with maximum plasma concentrations Cmax ranging from 35 to 55 mg/L being attained within 0.5 to 1 hour in healthy volunteers after oral administration of a single 500 mg dose .
Toxicity
A number of case reports demonstrating the capability for bendazac oral and eye drop therapy to cause potential hepatotoxicity via increases in serum transaminases in patients have been documented . Moreover, bendazac has also since been discontinued or withdrawn in various international regions due to the possibility and risk of eliciting hepatotoxicity in patients .
Chemical properties
White Solid
Originator
Versus,Angelini,Italy,1970
The Uses of Bendazac
Bendazac is a non-steroidal anti-inflammatory drug (NSAID). Bendazac is often used for joint and muscular pain. Bendazac has potential as an anti-denaturant drug for cataract and other condensation di seases as it protects proteins from denaturation induced by different agents.
Indications
Prior to the withdrawal of bendazac from various international regions of use due to concerns for hepatotoxicity the chemical had demonstrated potential usefulness predominantly as the prescription medication bendazac lysine for the indication of managing the level of vision in patients with mild to moderate cataracts to facilitate delaying the need for surgical intervention .
Elsewhere bendazac may still be available in a limited capacity as a non-prescription topical cream product for treating conditions like local pain, inflammation, dermatitis, eczema, pruritis, hives, insect bites, burns, erythema, and others - although such products may also be facing general discontinuation .
What are the applications of Application
Bendazac is inhibits the denaturing of proteins
Background
Bendazac is an oxyacetic acid . Despite possessing anti-inflammatory, anti-necrotic, choleretic, and anti-lipidemic characteristics, most research has revolved around studying and demonstrating the agent's principal action in inhibiting the denaturation of proteins - an effect that has primarily proven useful in managing and delaying the progression of ocular cataracts . Bendazac, however, has since been withdrawn or discontinued in various international regions due to its capability or risk for eliciting hepatotoxicity in patients although a small handful of regions may continue to have the medication available for purchase and use either as a topical anti-inflammatory/analgesic cream or eye drop formulation.
Definition
ChEBI: A monocarboxylic acid that is glycolic acid in which the hydrogen attached to the 2-hydroxy group is replaced by a 1-benzyl-1H-indazol-3-yl group. Although it has anti-inflammatory, antinecrotic, choleretic and antilipidaemic properties nd has been used for the treatment of various inflammatory skin disorders, its principal effect is to inhibit the denaturation of proteins. Its lysine salt is used in the management of cataracts.
Manufacturing Process
11 grams of the sodium salt of 1-benzyl-3-oxy-indazole are dissolved in 70 ml
of absolute ethanol by heating the resulting solution to boiling and stirring.
3.5 grams of chloroacetonitrile dissolved in 5 ml of absolute ethanol are then
added within 2-3 minutes and after 10 minutes a further portion of 1.7 grams
of chloroacetonitrile are added. The reaction is finally brought to completion
with an additional 45 minutes of boiling. The reaction mixture is allowed to
cool at room temperature and is then filtered. The alcohol solution is
evaporated to dryness under reduced pressure; the resulting residue is taken
up again with ether and the ether solution is washed in sequence with dilute
HCl, water, NaOH and water. The solution is dried on Na2SO4 and then the
solvent is removed. The residue consists of (1-benzyl-indazole-
3)oxyacetonitrile which is crystallized from methanol. It has a melting point of
93°C.
1 gram of the (1-benzyl-indazole-3)oxyacetonitrile is pulverized and is added
with stirring to 5 ml concentrated HCl. By heating on a boiling water bath for
2-3 minutes, the nitrile product melts and soon thereafter solidifies. The
precipitate is cooled, then filtered and washed well in a mortar with water.
After dissolution in 10% Na2CO3 it is precipitated again with dilute HCl. After crystallization from ethanol, 1-benzyl-indazole-3-oxyacetic acid is obtained. It
has a melting point of 160°C.
Therapeutic Function
Antiinflammatory
Pharmacokinetics
Bendazac principally demonstrates an antidenaturant action on proteins . This effect has been shown to inhibit the denaturation of various proteins like ocular lens proteins by heat, ultraviolet radiation, free radicals, and other chemicals . The medication may be administered to patients via a number of different formulations, including orally as the lysine salt, as eye drops, or even topical applications for the skin .
Some preliminary studies have suggested that an apparent improvement of the blood-retinal barrier had been observed in diabetic patients using bendazac lysine 500 mg three times a day for three to six months . Moreover, the use of topical bendazac has also been shown to demonstrate anti-inflammatory effects in animal models and clinical studies to effectively treat varied dermatoses, especially those involving a necrotic component .
Additionally, bendazac has also demonstrated choleretic and antilipidaemic activities that have resulted in substantial reductions in beta/alpha lipoprotein ratio, and total lipid, total cholesterol, and triglyceride levels in patients with dyslipidaemia using oral bendazac lysine 500 mg three times daily . The medication has also elicited the inhibition of phytohaemagglutinin induced lymphocyte transformation in vitro .
Safety Profile
Poison by intravenous andintraperitoneal routes. Moderately toxic by ingestion andsubcutaneous routes. When heated to decomposition itemits toxic fumes of NOx.
Metabolism
Bendazac is largely eliminated by metabolism, where more than 60% of an administered dose is excreted in the urine as the hydroxylated primary metabolite 5-hydroxybendazac and its glucuronide while up to approximately 15% of a bendazac dose is also excreted in the urine unchanged and as a glucuronide . Unfortunately, there is little data available regarding the specific enzymes responsible for bendazac's metabolism .
Properties of Bendazac
| Melting point: | 161-163°C |
| Boiling point: | 424.91°C (rough estimate) |
| Density | 1.1658 (rough estimate) |
| refractive index | 1.6240 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly, Heated) |
| form | Solid |
| pka | 2.89±0.10(Predicted) |
| color | Crystals from ethanol |
| λmax | 306nm(lit.) |
| Merck | 14,1035 |
| CAS DataBase Reference | 20187-55-7(CAS DataBase Reference) |
Safety information for Bendazac
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P501:Dispose of contents/container to..… |
Computed Descriptors for Bendazac
| InChIKey | BYFMCKSPFYVMOU-UHFFFAOYSA-N |
New Products
4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION Methylcobalamin (vitamin B12) SODIUM METHYL PARABEN SODIUM VALPROATE AMOXICILLIN (AMOXYCILLIN) TRIHYDRATE ACICLOVIRRelated products of tetrahydrofuran


You may like
-
 Bendazac 98% CAS 20187-55-7View Details
Bendazac 98% CAS 20187-55-7View Details
20187-55-7 -
 Bendazac CAS 20187-55-7View Details
Bendazac CAS 20187-55-7View Details
20187-55-7 -
 Bendazac CAS 20187-55-7View Details
Bendazac CAS 20187-55-7View Details
20187-55-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4