Azelastine
- CAS NO.:58581-89-8
- Empirical Formula: C22H24ClN3O
- Molecular Weight: 381.9
- MDL number: MFCD00242781
- EINECS: 253-720-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34
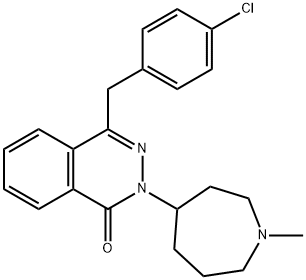
What is Azelastine?
Absorption
Systemic bioavailability of azelastine hydrochloride following intranasal administration is approximately 40%, reaching Cmax within 2-3 hours. When administered at doses greater than the recommended maximum, greater than proportional increases in both Cmax and AUC were observed.
Toxicity
Overdosage of intranasal or ophthalmic azelastine is unlikely to result in clinically significant adverse effects aside from increased drowsiness. If overdose does occur, employ general supportive measures. Oral ingestion of antihistamines, including non-oral formulations of azelastine, can cause serious adverse effects in children - for this reason, these products should be kept out of the reach of children. The oral LD50 in rats is 580 mg/kg.
Description
Azelastine has potent, long-lasting histamine H1-receptor blocking activity and several other interesting anti-inflammatory properties related to its antiallergic efficacy .
The Uses of Azelastine
anesthetic, anticonvulsant
Indications
Intranasal azelastine is indicated for the symptomatic treatment of seasonal allergic rhinitis in patients 5 years and older and for the symptomatic treatment of vasomotor rhinitis in patients 12 years and older. Ophthalmic azelastine solution is indicated for the treatment of itchy eyes associated with allergic conjunctivitis.
As a 0.15% nasal spray, azelastine hydrochloride is also indicated for over-the-counter treatment of allergies in patients aged six years and older.
Background
Azelastine, a phthalazine derivative, is an antihistamine available as an intranasal spray for the treatment of allergic and vasomotor rhinitis and as an ophthalmic solution for the treatment of allergic conjunctivitis. It is a racemic mixture, though there is no noted difference in pharmacologic activity between enantiomers, and was first granted FDA approval in 1996. Azelastine is also available in combination with fluticasone propionate as a nasal spray marketed under the trade name Dymista?, which is indicated for the symptomatic treatment of seasonal allergic rhinitis in patients 6 years of age and older.
Definition
ChEBI: A phthalazine compound having an oxo substituent at the 1-position, a 1-methylazepan-4-yl group at the 2-position and a 4-chlorobenzyl substituent at the 4-position.
brand name
Astelin (Medpointe); Optivar (Medpointe).
Pharmacokinetics
Azelastine antagonizes the actions of histamine, resulting in the relief of histamine-mediated allergy symptoms. Onset of action occurs within 15 minutes with intranasal formulations and as quickly as 3 minutes with ophthalmic solutions. Intranasal formulations have a relatively long-duration of action, with peak effects observed 4-6 hours after the initial dose and efficacy maintained over the entirety of the standard 12 hour dosing interval.
Clinical Use
Azelastine, although not a close structural analogue to the benzimidazoles, has some structural similarities to them. It is used as a nasal spray for allergic rhinitis and as eye drops for allergic conjunctivitis.Like olopatadine, azelastine also stabilizes mast cells, preventing degranulation and subsequent release of histamine, leukotrienes, and PGD2. It is available in Europe for systemic use in the treatment of asthma and seasonal allergies. Besides antihistaminic effects, it also may block release of histamine and other inflammatory mediators from mast cells. When administered orally, the N-dealkylated metabolite appears to contribute significantly to its pharmacological effects.
Safety Profile
Poison by ingestion,subcutaneous, intravenous, and intraperitoneal routes.Experimental reproductive effects. When heated todecomposition it emits toxic fumes of NOx and Cl- .
Metabolism
Azelastine hydrochloride is oxidatively metabolized to its main, and biologically active, metabolite desmethylazelastine by the cytochrome P450 enzyme system. Though labels for azelastine state that specific CYP enzyme involvement has not been elucidated, it has been suggested that the N-demethylation of azelastine is primarily catalyzed by CYP3A4, CYP2D6, and CYP1A2.
Properties of Azelastine
| Melting point: | 111 - 115°C |
| Boiling point: | 533.9±60.0 °C(Predicted) |
| Density | 1.1505 (rough estimate) |
| refractive index | 1.5790 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), DMSO (Slightly, Heated), Methanol (Slightly) |
| pka | pKa 9.82(H2O t=25 I=0.025) (Uncertain) |
| form | Solid |
| color | White to Off-White |
| Stability: | Stable under recommended storage conditions., Stable Under Recommended Storage C |
| CAS DataBase Reference | 58581-89-8(CAS DataBase Reference) |
Safety information for Azelastine
Computed Descriptors for Azelastine
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 Azelastine 98%View Details
Azelastine 98%View Details -
 58581-89-8 98%View Details
58581-89-8 98%View Details
58581-89-8 -
 Azelastine 95% CAS 58581-89-8View Details
Azelastine 95% CAS 58581-89-8View Details
58581-89-8 -
 0.05% Azelast Azelastine Eye Drops, 6 mlView Details
0.05% Azelast Azelastine Eye Drops, 6 mlView Details
58581-89-8 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
