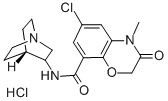
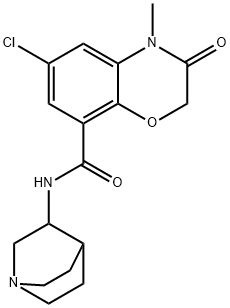
Azasetron hydrochloride
Synonym(s):6-Chloro-4-methyl-3-oxo-N-(quinuclidin-3-yl)-3,4-dihydro-2H-benzo[b][1,4]oxazine-8-carboxamide hydrochloride;N-(1-Azabicyclo[2.2.2]oct-3-yl)-6-chloro-4-methyl-3-oxo-3,4-dihydro-2H-1,4-benzoxazine-8-carboxamide hydrochloride;N-1-Azabicyclo[2.2.2]oct-3-yl-6-chloro-3,4-dihydro-4-methyl-3-oxo-2H-1,4-benzoxazine-8-carboxamide monohydrochloride;
- CAS NO.:123040-16-4
- Empirical Formula: C17H21Cl2N3O3
- Molecular Weight: 386.27
- MDL number: MFCD00209913
- EINECS: 692-159-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is Azasetron hydrochloride?
Description
Nazasetron (azasetron) hydrochloride is the fourth in the class of highly potent 5- HT3 receptor antagonists to reach the market for prophylaxis of severe nausea and vomiting induced by cytotoxic chemotherapy and by total body X-radiation. In a study with 146 cancer patients suffering from emesis induced by cisplatin, nazasetron was reported to be markedly effective in 72% of subjects. Nazasetron is devoid of dopamine D2 receptor blocking activity and therefore is free of the extrapyrimidal side effects associated with other commonly used antiemetics such as metoclopramide and domperidone. Nazasetron has been reported to be potentially useful for the treatment of gastrointestinal motility dysfunction, such as gastric stasis, vomiting and irritable bowel syndrome.
Chemical properties
White to Off-White Solid
Originator
Yoshitomi (Japan)
The Uses of Azasetron hydrochloride
Azasetron HCl is a selective 5-HT3 receptor antagonist with IC50 of 0.33 nM used in the management of nausea and vomiting induced by cancer chemotherapy.
The Uses of Azasetron hydrochloride
A 5-HT3 receptor antagonist. Used as an antiemetic.
What are the applications of Application
Azasetron Hydrochloride is a 5-HT3 receptor antagonist
What are the applications of Application
Y-25130 Hydrochloride is a potent and selective SR-3 antagonist
Definition
ChEBI: Azasetron hydrochloride is a benzoxazine.
Manufacturing Process
To a solution of 2-(2-carboxy-4-chlorophenoxy)acetic acid in concentrated
sulfuric acid is added dropwise a mixed liquid of fuming nitric acid and
concentrated sulfuric acid under stirring with keeping at a temperature below
10°C. After the addition, the reaction mixture is stirred and poured into 10 L
of ice-cold water. The precipitated crystals are collected by filtration, washed
with 2 L of water four times and them dried to give 2-(2-carboxy-4-chloro-6-
nitrophenoxy)acetic acid.
To a solution of ferrous sulfate heptahydrate in of hot water is added a
solution of 2-(2-carboxy-4-chloro-6-nitrophenoxy)acetic acid and aqueous
concentrated ammonia solution in water under stirring. After stirring, to the
reaction mixture is twice added aqueous concentrated ammonia solution.
While the reaction mixture becomes exothermic, stirring is continued. The
resultant mixture is filtered through a celite layer under reduced pressure and
washed with hot water twice. The filtrate is cooled and made acid with
concentrated hydrochloric acid. The precipitated crystals are washed with
water and dried to give 6-chloro-3,4-dihydro-3-oxo-2H-1,4-benzoxazine-8-
carboxylic acid.
A mixture of 6-chloro-3,4-dihydro-3-oxo-2H-1,4-benzoxazine-8-carboxylic
acid, methanol and concentrated sulfuric acid is refluxed under heating with
stirring, and then cooled. The precipitated crystals are collected by filtration,
washed with methanol and dried to give methyl 6-chloro-3,4-dihydro-3-oxo-
2H-1,4-benzoxazine-8-carboxylate, melting point 239°-241°C.
To a solution of methyl 6-chloro-3,4-dihydro-3-oxo-2H-1,4-benzoxazine-8-carboxylate in dimethylformamide is added potassium t-butoxide and solution
stirred at room temperature. To the resultant solution is added dropwise a
solution of methyl iodide in dimethylformainide under stirring. After the
reaction solution is stirred, water is added thereto. The insoluble substance is
collected by filtration, washed with water and dried to give methyl 6-chloro-
3,4-dihydro-4-methyl-3-oxo-2H-1,4-benzoxazine-8-carboxylate.
A mixture of methyl 6-chloro-3,4-dihydro-4-methyl-3-oxo-2H-1,4-
benzoxazine-8-carboxylate, ethanol and 4% aqueous potassium hydroxide
solution is refluxed with heating. The resultant solution is cooled and water is
added thereto followed by filtration. The filtrate is made acid with
concentrated hydrochloric acid. The precipitated crystals are collected by
filtration, washed with water and dried, and then recrystallized from ethanol
to give 6-chloro-3,4-dihydro-4-methyl-3-oxo-2H-1,4-benzoxazine-8-carboxylic
acid, melting point 241°-243°C.
A solution of 6-chloro-3,4-dihydro-4-methyl-3-oxo-2H-1,4-benzoxazine-8-
carboxylic acid in tetrahydrofuran and dimethylformamide is cooled to below
0°C and triethylamine is added under stirring thereto. Further, ethyl
chlorocarbonate is added and the mixture is stirred at room temperature. To
the resultant mixture is added 3-amino-8-azabicyclo[3.2.1]octane and the
mixture stirred. After completion of the reaction, aqueous sodium hydrogen
carbonate and ethyl acetate are added. The organic layer is separated,
washed with water and dried over magnesium sulfate. The solvent is distilled
off to give 6-chloro-3,4-dihydro-4-methyl-N-(8-azabicyclo[3.2.1]oct-3-yl)-3-
oxo-2H-1,4-benzoxazine-8-carboxamide.
In practice it is usually used as hydrochloride.
brand name
Serotone
Therapeutic Function
Antiemetic
Biological Activity
A selective and potent 5-HT 3 antagonist (K i = 2.9 nM).
storage
Desiccate at -20°C
Properties of Azasetron hydrochloride
| Melting point: | 281° (dec); mp 305° (dec) (Kawakita) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Methanol (Slightly), Water (Slightly) |
| form | White solid. |
| color | White |
| CAS DataBase Reference | 123040-16-4(CAS DataBase Reference) |
Safety information for Azasetron hydrochloride
Computed Descriptors for Azasetron hydrochloride
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 123040-16-4 Azasetron 98%View Details
123040-16-4 Azasetron 98%View Details
123040-16-4 -
 Azasetron hydrochloride CAS 123040-16-4View Details
Azasetron hydrochloride CAS 123040-16-4View Details
123040-16-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1