AURAPTENE
Synonym(s):7-[[(2E)-3,7-dimethyl-2,6-octadien-1-yl]oxy]-2H-1-benzopyran-2-one;7-Geranyloxycoumarin;Aurapten
- CAS NO.:495-02-3
- Empirical Formula: C19H22O3
- Molecular Weight: 298.38
- MDL number: MFCD00075948
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
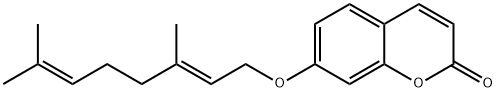
What is AURAPTENE?
Description
Auraptene (495-02-3) is a bioactive terpenoid occurring in a variety of citrus fruits and possessing therapeutic potential.1?? Displays neuritogenic activity2?and neuroprotective effects via suppression of inflammation and induction of GDNF and BDNF in neuronal cells3. Attenuates ROS production and enhances mitochondrial respiration which mitigates Parkinson’s disease-like behavior.4?Displays hepatoprotective5?and chemopreventive activity6.
The Uses of AURAPTENE
Auraptene is a natural bioactive monoterpene coumarin ether. Auraptene has shown a remarkable effect in the prevention of degenerative diseases.
The Uses of AURAPTENE
antineoplastic, apoptosis inducer
What are the applications of Application
Auraptene is a cell proliferation and ACAT inhibitor and PPAR agonist
Definition
ChEBI: Auraptene is a member of the class of coumarins that is umbelliferone in which the phenolic hydrogen has been replaced by a geranyl group. Ii is isolated from several edible fruits and vegetables and exhibits a variety of therapeutic properties. It has a role as a plant metabolite, an antineoplastic agent, an apoptosis inducer, a dopaminergic agent, a neuroprotective agent, an antihypertensive agent, a gamma-secretase modulator, a vulnerary, an EC 2.7.11.24 (mitogen-activated protein kinase) inhibitor, a PPARalpha agonist, a gastrointestinal drug, a matrix metalloproteinase inhibitor, an antioxidant and a hepatoprotective agent. It is a member of coumarins and a monoterpenoid. It is functionally related to an umbelliferone.
References
1) Bibak et al. (2019), A Review of the Pharmacological and Therapeutic Effects of Auraptene; Biofactors, 45 867 2) Furukawa et al. (2012), Neurotrophic Effects of Citrus Auraptene: Neuritogenic Activity in PC12 Cells; Int. J. Mol. Sci., 13 5338 3) Furukawa et al. (2020), Citrus Auraptene Induces Expression of Brain-Derived Neurotrophic Factor in Neuro2a Cells; Molecules, 25 1117 4) Jang et al. (2019), Auraptene Mitigates Parkinson’s Disease-Like Behavior by Protecting Inhibition of Mitochondrial Respiration and Scavenging Reactive Oxygen Species; Int. J. Mol. Sci., 20 3409 5) Wang et al. (2019), Hepatoprotection of Auraptene From the Peels of Citrus Fruits Against 17α-ethinylestradiol-induced Cholestasis in Mice by Activating Farnesoid X Receptor; Food Funct., 10 3839 6) Tanaka et al. (1998), Citrus Auraptene Exerts Dose-Dependent Chemopreventative Activity in Rat Large Bowel Tumorigenesis: The Inhibition Correlates With Suppression of Cell Proliferation and Lipid Peroxidation and With Induction of Phase II Drug-Metabolizing Enzymes; Cancer Res., 58 2550
Properties of AURAPTENE
| Melting point: | 91 °C |
| Boiling point: | 455.5±45.0 °C(Predicted) |
| Density | 1.079±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | DMSO: >20mg/mL |
| form | powder |
| color | white to off-white |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
Safety information for AURAPTENE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for AURAPTENE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium 3-Bromopyrazole (3aR,4R,5R,6aS)-hexahydro-5-Triethyl silyloxy-4-((E)-3-oxo-5-phenylpent-1- enyl)cyclopenta[b]furan-2-one. 1-Chlorocarbonyl-4-piperidinopiperidine 1-Bromo-4-phenyl-2-Butanone 4-Amino-2-fluoro-N-methylbenzamide 1,1'-Carbonyldiimidazole SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTIONRelated products of tetrahydrofuran

You may like
-
 Auraptene 95% CAS 495-02-3View Details
Auraptene 95% CAS 495-02-3View Details
495-02-3 -
 Auraptene CAS 495-02-3View Details
Auraptene CAS 495-02-3View Details
495-02-3 -
 Aurapten CAS 495-02-3View Details
Aurapten CAS 495-02-3View Details
495-02-3 -
 Auraptene CAS 495-02-3View Details
Auraptene CAS 495-02-3View Details
495-02-3 -
![Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
54094-19-8 -
 85-81-4 99%View Details
85-81-4 99%View Details
85-81-4 -
![208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 -
 Meldrums acid 2033-24-1 99%View Details
Meldrums acid 2033-24-1 99%View Details
2033-24-1