Atipamezole
Synonym(s):4-(2-Ethyl-2,3-dihydro-1H-inden-2-yl)-1H-Imidazole;Antisedan;MPV 1248
- CAS NO.:104054-27-5
- Empirical Formula: C14H16N2
- Molecular Weight: 212.29
- MDL number: MFCD00864502
- EINECS: 1806241-263-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
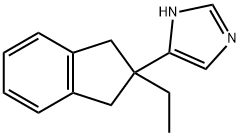
What is Atipamezole?
Originator
Antisedan,Novartis
The Uses of Atipamezole
Atipamezole has been used as a α2-adrenoceptor antagonist in mesencephalic trigeminal nucleus (MTN) neurons, CD4+ T-lymphocyte and human embryonic kidney (HEK293) membrane preparation.
The Uses of Atipamezole
Antagonist (α2-receptor).
Manufacturing Process
(a). 2-Acetyl-1-indanone (Liebigs Ann. Chem. 1906, 347, 112) is alkylated
with ethylbromide in acetone in the presence of sodium carbonate to 2-acetyl-
2-ethyl-1-indanone. The acetyl group is brominated with bromine in methanol
and to imidazole by heating in formamide as before. The melting point of the
4(5)-(2,3-dihydro-2-ethyl-1-oxo-1H-inden-2-yl)imidazole is 126-127°C (from
ethyl acetate).
(b). The carbonyl group of 4(5)-(2,3-dihydro-2-ethyl-1-oxo-1H-inden-2-
yl)imidazole is reduced to the alcohol group with sodium borohydride in
ethanol. The product is the mixture of cis-trans stereoisomers, the purification
of which is accomplished by liquid chromatography: cis-isomer as
hydrochloride (melting point 184-185°C), 1H NMR (80 MHz, MeOH-d4): 0.73
(3H, t), 1.86 (2H, m), 3.36 (2H, m), 3.61 (3H, s), 5.15 (1H, s), 7.06 (1H, d),
7.2-7.4 (4H, m), 8.69 (1H, d), and trans-isomer as hydrochloride, 1H NMR
(80 MHz, MeOH-d4): 0.80 (3H, t), 1.84 (2H, m), 3.15 (2H, m), 3.24 (3H, s),
5.15 (1H, s), 6.87 (1H, d), 7.2-7.4 (4H, m), 8.54 (1H, d).
The oxo derivative prepared in step (a) or the hydroxy derivative prepared in
step (b) is hydrogenated in 2 N hydrochloric acid in the presence of 10%
palladium on carbon at 70°C. When the uptake of hydrogen ceases, the
reaction mixture is filtered and made alkaline. The product is extracted with
methylene chloride which is washed with water, dried and evaporated to
dryness. From the residue, which is the product as base, is made the
hydrochloride of 4(5)-(2,3-dihydro-2-ethyl-1H-inden-2-yl)imidazole using dry
hydrogen chloride in ethyl acetate. It has melting point 211-215°C.
brand name
Antisedan (Farmos Group Ltd., Finland).
Therapeutic Function
Antihypertensive
General Description
Atipamezole has an imidazole structure and gets localized in the central nervous system on administration.
Biochem/physiol Actions
Atipamezole is a selective α2 adrenergic blocker. Atipamezole is more potent than yohimbine; it is very selective for α2 adrenergic vs α1 sites, but not selelctive for α2 subtypes.
Properties of Atipamezole
| Melting point: | 126-129oC |
| Boiling point: | 367.1±11.0 °C(Predicted) |
| Density | 1.115±0.06 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | DMSO: ≥30mg/mL |
| pka | 14.05±0.10(Predicted) |
| form | powder |
| color | white to brown |
| InChI | InChI=1S/C14H16N2/c1-2-14(13-9-15-10-16-13)7-11-5-3-4-6-12(11)8-14/h3-6,9-10H,2,7-8H2,1H3,(H,15,16) |
| CAS DataBase Reference | 104054-27-5(CAS DataBase Reference) |
Safety information for Atipamezole
Computed Descriptors for Atipamezole
| InChIKey | HSWPZIDYAHLZDD-UHFFFAOYSA-N |
| SMILES | C1NC(C2(CC)CC3=C(C2)C=CC=C3)=CN=1 |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Atipamezole CAS 104054-27-5View Details
Atipamezole CAS 104054-27-5View Details
104054-27-5 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1