Gilteritinib
- CAS NO.:1254053-43-4
- Empirical Formula: C29H44N8O3
- Molecular Weight: 552.71
- MDL number: MFCD28144685
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
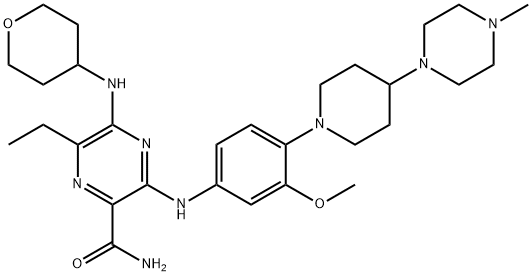
What is Gilteritinib?
Absorption
In preclinical trials, the maximal plasma concentration of gilteritinib was observed 2 hours after oral administration and followed by a maximal intratumor concentration after 4-8 hours. The maximum concentration, as well as the AUC, were modified correspondingly with the dose and were reported to be 374 ng/ml and 6943 ng.h/ml, respectively. The steady-state plasma level is reached within 15 days of dosing with an approximate 10-fold bioaccumulation.
In a fasted state in humans, the tmax is reported to be of 4-6 hours. The Cmax and AUC were decreased by 26% and 10% respectively by the co-ingestion of a high-fat meal with a tmax delay of 2 hours.
Toxicity
Gilteritinib is not reported to be mutagenic in bacterial mutagenesis assays nor clastogenic in aberration test assays in Chinese hamster lung cells. However, it resulted positive for the induction of micronuclei in mouse bone marrow and for the degeneration and necrosis of germ cells and spermatid giant cell formation in testis as well as single cell necrosis of the epididymal duct epithelia.
Description
Gilteritinib is an inhibitor of FMS-related tyrosine kinase 3 (FLT3; IC50 = 5 nM for the wild-type enzyme). It inhibits mutant forms of FLT3, including FLT3 with the internal tandem duplication mutation (FLT3-ITD), FLT3-ITD expressing the F691L mutation, and FLT3 with various tyrosine kinase domain mutations (FLT3-TKD; IC50s =1.4-1.8, 12.2, and 0.7-2 nM, respectively). Gilteritinib also inhibits Axl and c-Kit with IC50 values of 41 and 102 nM, respectively. It decreases the viability of blast cells isolated from patients with relapsed acute myeloid leukemia (AML) in a concentration-dependent manner. Formulations containing gilteritinib have been used in the treatment of AML.
The Uses of Gilteritinib
Gilteritinib is an AXL inhibitor in cancer.
Indications
Gilteritinib is indicated for the treatment of adult patients who have relapsed or refractory acute myeloid leukemia with an FLT3 mutation detected by an FDA-approved test. This indication was expanded for a companion diagnostic to include use with gilteritinib such as the LeukoStrat CDx FLT3 Mutation Assay.
Acute myeloid leukemia is cancer that impacts the blood and bone marrow with a rapid progression. This condition produces low numbers of normal blood cells and the requirement of continuous need for transfusions.
Background
Gilteritinib, also known as ASP2215, is a small molecule part of the FLT3 tyrosine kinase inhibitors that presented a greater selectivity and potency when compared with other agents from this group. It is a pyrazinecarboxamide derivative that showed high selectivity to FLT3 preventing the c-Kit -driven myelosuppression observed in other therapies. Gilteritinib was developed by Astellas Pharma and FDA approved on November 28, 2018. This drug was approved after being designed as an orphan drug with a fast track and priority review status.
Definition
ChEBI: Gilteritinib is a member of the class of pyrazines that is pyrazine-2-carboxamide which is substituted by {3-methoxy-4-[4-(4-methylpiperazin-1-yl)piperidin-1-yl]phenyl}nitrilo, (oxan-4-yl)nitrilo and ethyl groups at positions 3,5 and 6, respectively. It is a potent inhibitor of FLT3 and AXL tyrosine kinase receptors (IC50 = 0.29 nM and 0.73 nM, respectively). Approved by the FDA for the treatment of acute myeloid leukemia in patients who have a FLT3 gene mutation. It has a role as an apoptosis inducer, an EC 2.7.10.1 (receptor protein-tyrosine kinase) inhibitor and an antineoplastic agent. It is a N-methylpiperazine, a member of piperidines, a secondary amino compound, a monomethoxybenzene, a member of pyrazines, a primary carboxamide, an aromatic amine and a member of oxanes.
brand name
Xospata
General Description
Class: receptor tyrosine kinase; Treatment: AML, SM; Other name: ASP-2215; Oral bioavailability > 61%; Elimination half-life = 113 h; Protein binding = 94%
Pharmacokinetics
In preclinical trials, gilteritinib demonstrate an IC50 for the wild-type receptor of 5 nM, 0.7-1.8 nM for ITD-mutated and comparable inhibition to other therapies in the TKD-mutated. As well, data showed a gilteritinib-driven inhibition of the receptor tyrosine kinase AXL which is known to modulate the activity of FLT3 in acute myeloid leukemia. Another important result in vivo was the localization in high levels in xenografted tumors which indicated high selectivity.
In phase 1/2 clinical trials, gilteritinib was shown to present a composite complete response of 41%, an overall response rate of 52%, a median duration of response of 20 weeks with a median overall survival of 31 weeks.
In phase III clinical trials, gilteritinib reported a complete remission or complete remission with partial hematologic recovery in 21% of the patients.
Metabolism
Gilteritinib is primarily metabolized in the liver by the activity of CYP3A4. Its metabolism is driven by reactions of N-dealkylation and oxidation which forms the metabolite M17, M16 and M10. From the plasma concentration, the major form is the unchanged drug.
References
Lee et al. (2017) Preclinical studies of gilteritinib, a next-generation FLT3 inhibitor; Blood?129 257 Thomas et al. (2021) Gilteritinib Inhibits Glutamine Uptake and Utilization in FLT-3-ITD-Positive AML; Cancer Ther.?20 2207
Properties of Gilteritinib
| Melting point: | >157°C (dec.) |
| Boiling point: | 696.9±55.0 °C(Predicted) |
| Density | 1.242±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (25 mg/ml) |
| form | solid |
| pka | 14.39±0.50(Predicted) |
| color | Off-white to pale pink |
| Stability: | Stable for 1 year as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
Safety information for Gilteritinib
Computed Descriptors for Gilteritinib
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![N2-[2-Methoxy-4-[4-(4-methyl-1-piperazinyl)-1-piperidinyl]phenyl]-N4-[2-[(1-methylethyl)sulfonyl]phenyl]-1,3,5-triazine-2,4-diamine](https://img.chemicalbook.in/CAS/GIF/1097917-15-1.gif)
![N-(1,1-Dimethylethyl)-3-[[5-methyl-2-[[4-(4-methyl-1-piperazinyl)phenyl]amino]-4-pyrimidinyl]amino]benzenesulfonamide](https://img.chemicalbook.in/CAS2/GIF/936091-14-4.gif)

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1