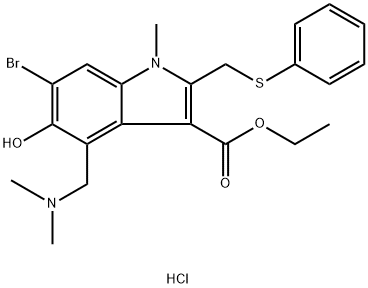
Arbidol hydrochloride
Synonym(s):6-Bromo-4-((dimethylamino)methyl)-5-hydroxy-1-methyl-2-((phenylthio)methyl)-1H-Indole-3-carboxylic acid ethyl ester monohydrochloride
- CAS NO.:131707-23-8
- Empirical Formula: C22H26BrClN2O3S
- Molecular Weight: 513.88
- MDL number: MFCD00344795
- EINECS: 680-680-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
What is Arbidol hydrochloride?
Description
Umifenovir is a broad-spectrum antiviral agent. It inhibits the replication of H5N1 influenza (IC50 = 30 μg/ml) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in vitro (IC50 = 3.537 μM). Umifenovir inhibits entry of hepatitis C virus (HCV) pseudoparticles in Huh7 cells (IC50 = 6 μg/ml) and inhibits Zika virus protein synthesis in Vero cells. In vivo, umifenovir (45 mg/kg) reduces the formation of influenza-induced lung lesions in ferrets.
Chemical properties
Off White to Pale Yellow Solid
The Uses of Arbidol hydrochloride
Arbidol hydrochloride (ARB) is a small monocular compound. It is used to prevent severe pneumonia and virus-associated cytokine dysregulation induced by influenza viruses (IFV). ARB also possesses some immunomodulatory properties, including the effects of interferon induction and macrophage activation.
Arbidol is a broad-spectrum antiviral that has demonstrated activity against a number of enveloped and non-enveloped viruses, and is used clinically to treat influenza. Arbidol inhibits viral entry into host cell and stimulates immune response. Arbidol inhibits fusion between the viral capsid and the cell membrane of the target cell, thus preventing viral entry.
The Uses of Arbidol hydrochloride
Arbidol is an antiviral treatment for influenza infection.
What are the applications of Application
Arbidol Hydrochloride is an antiviral agent
Biochem/physiol Actions
Arbidol hydrochloride (ARB) is a small monocular compound. It is used to prevent severe pneumonia and virus-associated cytokine dysregulation induced by influenza viruses (IFV). ARB also possesses some immunomodulatory properties, including the effects of interferon induction and macrophage activation.
Storage
Store at -20°C
References
1) Blasing?et al.?(2014), Arbidol as a broad-spectrum antiviral: an update; Antiviral Res,?107?84 2) Boriskin?et al. (2008) Arbidol: a broad-spectrum antiviral compound that blocks viral fusion; Curr. Med. Chem.,?15?997 3) Brooks?et al.?(2012) Antiviral activity of Arbidol, a broad-spectrum drug for use against respiratory viruses, varies according to test conditions; J. Med. Virol.,?84?170 4) Leneva?et al.?(2009)?Characteristics of Arbidol-resistant mutants of influenza virus: Implications for the mechanism of anti-influenza action of arbidol; Antiviral Res.,?81?132 5) Wang?et al.?(2020)?The anti-influenza drug, arbidol, is an efficient inhibitor of SARS-CoV-2 in vitro; Cell Discov.,?6?28 6) Vankadari?(2020)?Arbidol: A Potential Antiviral Drug for the Treatment of SARS-CoV-2 by Blocking Trimerization of the Spike Glycoprotein; Int. J. Antimicrob. Agents, 56 105998
Properties of Arbidol hydrochloride
| Melting point: | 133-137?C |
| storage temp. | -20°C |
| solubility | DMSO: soluble20mg/mL protein, clear |
| form | powder |
| color | white to beige |
| Stability: | Hygroscopic |
| InChI | InChI=1S/C22H25BrN2O3S.ClH/c1-5-28-22(27)20-18(13-29-14-9-7-6-8-10-14)25(4)17-11-16(23)21(26)15(19(17)20)12-24(2)3;/h6-11,26H,5,12-13H2,1-4H3;1H |
| CAS DataBase Reference | 131707-23-8(CAS DataBase Reference) |
Safety information for Arbidol hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Arbidol hydrochloride
| InChIKey | OMZHXQXQJGCSKN-UHFFFAOYSA-N |
| SMILES | C(C1=C(N(C)C2=CC(Br)=C(O)C(CN(C)C)=C12)CSC1C=CC=CC=1)(=O)OCC.Cl |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran





You may like
-
 131707-23-8 Arbidol hydrochloride 98%View Details
131707-23-8 Arbidol hydrochloride 98%View Details
131707-23-8 -
 131707-23-8 98%View Details
131707-23-8 98%View Details
131707-23-8 -
 Arbidol hydrochloride CAS 131707-23-8View Details
Arbidol hydrochloride CAS 131707-23-8View Details
131707-23-8 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
