Arbekacin
- CAS NO.:51025-85-5
- Empirical Formula: C22H44N6O10
- Molecular Weight: 552.62
- MDL number: MFCD00864919
- EINECS: 204-767-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
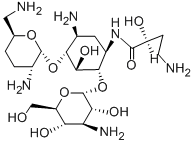
What is Arbekacin?
Description
Arbekacin is a semi-synthetic derivative of dibekacin useful in the treatment of bacterial infections. This aminoglycoside is active against a broad spectrum of bacteria, including some of the gentamycin-, kanamycin-, and tobramycin-resistant pathogens. Compared to amikacin and dibekacin, ototoxicity is reportedly milder.
Originator
Inst. Microbial Chemistry (Japan)
Definition
ChEBI: A kanamycin that is kanamycin B bearing an N-(2S)-4-amino-2-hydroxybutyryl group on the aminocyclitol ring.
Manufacturing Process
13.53 g (30 mmole) of 3',4'-dideoxykanamycin (abbreviated as DKB) in the
form of the free base was dissolved in 135 ml of water, and to this solution
was added dropwise 5.61 g (33 mmole) of benzyloxycarbonyl chloride over 1
h under stirring and under ice-cooling (0°-5°C). After the addition, the
mixture was further stirred for 1 h at room temperature and filtered to
remove the precipitate.
The filtrate was washed with 135 ml of ethyl ether. The aqueous phase was
neutralized by addition of aqueous ammonia and then concentrated under
reduced pressure. The concentrated solution was passed through a column of
480 ml of a cation-exchange resin essentially consisting of a copolymer of
methacrylic acid and divinylbenzene (available under a trade name "Amberlite CG 50", a product of Rohm and Haas Co., U.S.A. the ammonium form) to
effect the adsorption of the benzyloxycarbonylated DKB by the resin. The
resin column was washed with water (1920 ml) and then eluted with 0.1 N
aqueous ammonia. 960 ml of the first running of the eluate was discarded,
and the subsequently running fraction of the eluate amounting to 780 ml was
collected, concentrated and freeze-dried to give 5.43 g (yield 31%) of 6'-Nbenzyloxycarbonyl
DKB as a colorless powder, melting point 113°-115°C
(dec.).
4.04 g (6.9 mmole) of the 6'-N-benzyloxycarbonyl DKB was dissolved in 26 ml
of water, and to this solution was added a solution of 2.94 g (8 mmole) of Nhydroxysuccinimide
ester of (S)-4-benzyloxycarbonylamino-2-hydroxybutyric
acid in 45 ml of dimethoxyethane. The admixture was stirred at room
temperature for 90 min and then the reaction mixture was concentrated to
dryness. The residue was taken up in a volume of water and the aqueous
solution was poured into a column of 560.0 g of silica gel. The elution was
conducted using methanol-chloroform-17% aqueous ammonia (4:2:1), and
such eluate fractions containing the unreacted materials were discarded. The
fractions containing the mixed acylated products were collected and
concentrated to give 5.63 g of the mixed acylated products. The mixed
acylated products were dissolved in a mixture of 67 ml of glacial acetic acid,
63 ml of methanol and 17 ml of water, and the solution so obtained was
admixed with 1.6 g of 5% palladium-carbon and hydrogenated with hydrogen
at atmospheric pressure for 4 h to remove the benzyloxycarbonyl groups of
the acylated products. The reaction mixture was filtered to remove the
catalyst, and the filtrate was concentrated to under reduced pressure to give
5.20 g of a powder of the acetate of the acylated products comprising 1-N-
[(S)-4-amino-2-hydroxybutyryl] DKB acetate.
The aqueous solution of 1-N-[(S)-4-amino-2-hydroxybutyryl] DKB acetate was
poured into a column of 250 ml of a cation-exchange resin made of a
copolymer of methacrylic acid and divinylbenzene (commerically available as
"Amberlite CG 50" ammonium form). The resin column was washed with
water and eluted successively with aqueous ammonia (0.1 N 850 ml, 0.3 N
830 ml, 0.63 N 830 ml and 1 N 830 ml). The eluate was collected in 17 ml
fractions. 320 ml of the fractions which were eluted by using 1 N aqueous
ammonia and which showed high antibacterial activity to Bacillus subtilis PC
1219 and Escherichia coli JR 66/W 677 were combined together and
concentrated to dryness to give 301.0 mg of a powder. This powder was
rechromatographed again into a column of 11 ml of a cation-exchange resin
made of a copolymer of methacrylic acid with divinylbenzene (commercially
available as "Amberlite CG 50", ammonium form).
Thus, the resin column was at first washed with 40 ml of water and then with
90 ml of 0.5 N aqueous ammonia, and subsequently the elution was made
using 0.75 N aqueous ammonia. Such fractions of the eluate were combined
together to a total volume of 26 ml and concentrated to dryness to give 61.0
mg (yield 1.6%) of 1-N-[(S)-4-amino-2-hydroxybutyryl]DKB as a colorless
crystalline powder, melting point 178°C (dec.).
brand name
Habekacin
Therapeutic Function
Antibiotic
Antimicrobial activity
The 1-N-(4-amino-2-hydroxybutyryl) derivative of dibekacin,
to which it bears the same relation as amikacin bears to kanamycin
A. Supplied as the sulfate.
Activity and stability to aminoglycoside-modifying enzymes
are comparable with those of amikacin. It is active against
many strains of methicillin-resistant Staph. aureus, either alone
or in combination with β-lactam or other agents. Synergy with
ampicillin has been observed for high-level gentamicin- and
vancomycin-resistant enterococci.
A 3 mg/kg intravenous dose achieved a peak concentration
of c. 8 mg/L after 1 h. The plasma half-life is about 2 h and
protein binding 3–12%.
About 85% of the dose can be recovered from urine over
48 h. It is retained in renal failure, but moderately well removed
by hemodialysis with a plasma half-life of 2–4 h. Peak concentrations
of 10.9 mg/L and trough concentrations of 1.7 mg/L
have been reported in patients treated for MRSA infection
where Cmax:MIC ratios of >25 and AUC:MIC ratios of >186
were associated with improved cure rates, and both Cmin and
AUC were associated with the incidence of nephrotoxicity.
Toxicity and side effects are typical of the aminoglycoside
class. It is used in severe infection cause by susceptible microorganisms,
but is not widely available.
Properties of Arbekacin
| Boiling point: | 904.0±65.0 °C(Predicted) |
| Density | 1.47±0.1 g/cm3(Predicted) |
| pka | 13.07±0.70(Predicted) |
| CAS DataBase Reference | 51025-85-5(CAS DataBase Reference) |
Safety information for Arbekacin
Computed Descriptors for Arbekacin
Arbekacin manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8