Apraclonidine
- CAS NO.:66711-21-5
- Empirical Formula: C9H10Cl2N4
- Molecular Weight: 245.11
- MDL number: MFCD00865638
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
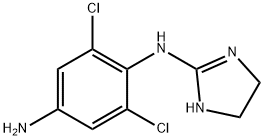
What is Apraclonidine?
Absorption
Topical use of apraclonidine ophthalmic solution leads to systemic absorption. Studies of apraclonidine (0.5% ophthalmic solution) dosed one drop three times a day in both eyes for 10 days in normal volunteers yielded mean peak and trough concentrations of 0.9 ng/mL and 0.5 ng/mL, respectively.
Toxicity
Accidental or intentional ingestion of oral apraclonidine has been reported to cause apnea, arrhythmias, asthenia, bradycardia, conduction defects, diminished or absent reflexes, dryness of the mouth, hypotension, hypothermia, hypoventilation, irritability, lethargy, miosis, pallor, respiratory depression, sedation or coma, seizure, somnolence, transient hypertension, and vomiting.
Originator
Alfadrops,Cipla Limited,India
Indications
For prevention or reduction of intraoperative and postoperative increases in intraocular pressure (IOP) before and after ocular laser surgery when used prophylactically. Also used as a short-term adjunctive therapy in patients with open-angle glaucoma who are on maximally tolerated medical therapy requiring additional IOP reduction.
Background
Apraclonidine, also known as iopidine, is a sympathomimetic used in glaucoma therapy. It is an alpha2-adrenergic agonist.
Definition
ChEBI: An imidazoline compound having a 4-amino-2,6-dichloroanilino group at the 2-position.
Manufacturing Process
The preparation of p-aminoclonidine (apraclonidine) consists of 6 steps.
In the first step 2,6-dichloro-4-nitroaniline was converted to 2,6-dicloro-4-
nitrophenylisothiocyanate by addition of thiophosgene in toluene according to
the method described in Great Britain Patent No.: 1,131,780 (Beck et al.).
The second step involved the conversation of 2,6-dichloro-4-nitrophenylisothiocyanate
to 1-(2-aminoethyl)-3-(2,6-dichloro-4-nitrophenyl)-thiourea
ethylenediamine solvate. The solution of 2,6-dicloro-4-nitrophenylisothiocyanate
(432 g, 1.73 mol) in 2 L of toluene was added dropwise to the
cooled (0°C) solution ethylenediamine (244 ml, 3.66 mol, 2.1 eq.) in toluene
(4 L) under a nitrogen atmosphere. 2-Propanol (1 L) was added and after 5
minutes, the solid was collected by filtration, washed with 20% 2-
propanol/toluene, and dried to a constant weight of 602 g (94%). This
product is hygroscopic, mp 120°C (dec.).
The third step was the conversation of 1-(2-aminoethyl)-3-(2,6-dichloro-4-
nitrophenyl)-thiourea ethylenediamine solvate to 2-[(2,6-dicloro-4-
nitrophenyl)imino]imidazoline ethylenediamine solvate. (500 g, 1.35 mol) of
above prepared thiourea solvate was suspended with toluene (4 L) and was
heated at reflux for 15 hours. The mixture was cooled to 23°C and 1 M
aqueous hydrochloric acid (4 L) was added. After stirring for 10 min the
biphasic mixture was filtered to remove a sticky insoluble material. The
aqueous phase was neutralized to pH=7.0 using 50% NaOH. After stirring for
1 hour the yellow solid was collected by filtration, washed with water (4 L)
and t-butyl methyl ether (2 L) and dried in air to constant weight of 195 g
(52%), m.p. 289-292°C.
The fourth step was the conversation of 2-[2,6-dichloro-4-nitrophenyl)
imino]imidazoline (150 g, 0.55 mol) in methanol (1,5 L) to 2-[(2,6-dichloro-4-
aminophenyl)imino]imidazoline by hydrogen with 30 g Raney nickel catalyst at
23°C for 22 hours. After removing the catalyst hydrogen chloride gas was
bubbled into solution until pH of the reaction mixture was 1.0. The solvent
was rotary removed in vacuum and the residual solid was slurried with 2-
propanol (1 L). The solvent was again removed by rotary evaporation, the
cream solid was triturated with 2-propanol (600 ml). After aging for 1 hour,
the solid was collected by filtration, washed with 2-propanol and t-butyl
methyl ether, and dried for 15 hours at 6°C and t-butyl methyl ether, and
dried for 15 hours at 60°C and 20 mm Hg. Yield of dihydrochloride 167 g (96%), mp 260°C (dec.).
The dihydrochloride was converted to the monochloride (step 5) by adding 5
M aqueous sodium hydroxide dropwise to pH=6.5 at 5°C for 2 hours. Yield of
hydrochloride 87%.
The last step was recrystallization of product from water. The recrystallized
material had m.p. 300°C. Calculated for: C9H10Cl2N4HCl: C, 38.39; H, 3.94;
N, 19.90; Cl, 37.78. Found: C, 38.36; H, 3.91; N, 19.83; Cl, 37.77.
Therapeutic Function
Antiglaucoma
General Description
In addition to its therapeutic use as an antihypertensiveagent, clonidine has been found to provide beneficialeffects in several other situations, including glaucoma,spasticity, migraine prophylaxis, opiate withdrawal syndrome,and anesthesia. This has prompted the developmentof analogs of clonidine for specific use in some of the mentionedareas. Two of such examples are apraclonidine andbrimonidine. Apraclonidine does not cross the BBB.However, brimonidine can cross the BBB and hence canproduce hypotension and sedation, although these CNSeffects are slight compared with those of clonidine. CNS effectsof these drugs are correlated well to their log P, pKa,and thus log D value. Both apraclonidine and brimonidineare selective α2-agonists with α1: α2 ratios of 30:1 and1,000:1, respectively. Brimonidine is a much more selectiveα2-agonist than clonidine or apraclonidine and is a firstlineagent for treating glaucoma. Apraclonidines’s primarymechanism of action may be related to a reduction of aqueousformation, whereas brimonidine lowers intraocularpressure by reducing aqueous humor production and increasinguveoscleral outflow. Apraclonidine is used specificallyto control elevations in intraocular pressure that canoccur during laser surgery on the eye. Another example istizanidine (Zanaflex), which finds use in treating spasticityassociated with multiple sclerosis or spinal cord injury. Bystimulating 2-adrenergic receptors, it is believed to decreasethe release of excitatory amino acid NTs from spinalcord interneurons.
Pharmacokinetics
Apraclonidine significantly lowers intraocular pressure with minimal effects on cardiovascular and pulmonary parameters. It lowers intraocular pressure by reducing aqueous humor production and increasing uveoscleral outflow.
Veterinary Drugs and Treatments
Apraclonidine is an alpha2 adrenergic agonist used to reduce aqueous humor secretion. Apraclonidine is a relatively selective, alphaadrenergic agonist and does not have significant membrane stabilizing (local anesthetic) activity. The onset of action is within 3 – 5 hours of a single dose. It apparently is less effective than brimonidine in dogs and is very potent, causing vomiting and diarrhea in cats and dogs. Apraclonidine will reduce aqueous production but must be combined with other agents for adequate control. Neither the beta-blockers nor alpha-agonists are as effective as the carbonic anhydrase inhibitors in decreasing aqueous production.
Metabolism
Not Available
Properties of Apraclonidine
| Melting point: | >230° |
| Boiling point: | 395.5±52.0 °C(Predicted) |
| Density | 1.63±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: 1.4 mg/mL Solutions may be stored for several days at 4°C |
| form | solid |
| pka | 9.26±0.50(Predicted) |
| color | white |
| CAS DataBase Reference | 66711-21-5(CAS DataBase Reference) |
Safety information for Apraclonidine
Computed Descriptors for Apraclonidine
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
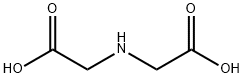
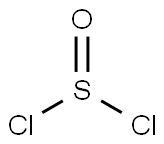
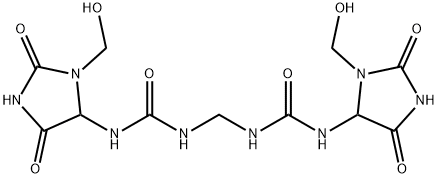


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8