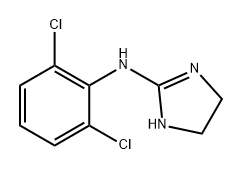
Clonidine hydrochloride
Synonym(s):2-(2,6-Dichloroanilino)-2-imidazoline hydrochloride;2-(4,6-Dichloro-2-methyl-1h-indol-3-yl)ethanamine;4,6-Dichloro-2-methyl-3-aminoethylindole
- CAS NO.:4205-91-8
- Empirical Formula: C9H10Cl3N3
- Molecular Weight: 266.55
- MDL number: MFCD00036705
- EINECS: 224-121-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
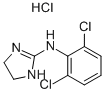
What is Clonidine hydrochloride?
Chemical properties
White Solid
Originator
Catapresan,Boehringer,Ingelheim,1966
The Uses of Clonidine hydrochloride
Clonidine hydrochloride tablets are indicated in the treatment of hypertension. It has found new uses, including treatment of some types of neuropathic pain, opioid detoxification, sleep hyperhidrosis, anaesthetic use, and off-label, to counter the side effects of stimulant medications such as methylphenidate or amphetamine. It is becoming a more accepted treatment for insomnia, as well as for relief of menopausal symptoms. Clonidine(4205-91-8) is increasingly used in conjunction with stimulants to treat attention-deficit hyperactivity disorder (ADHD).
The Uses of Clonidine hydrochloride
Labelled Clonidine. α2-Adrenergic agonist. Antihypertensive; analgesic for neuropathic pain.
The Uses of Clonidine hydrochloride
`a2-adrenoceptor agonist, imidazoline receptor ligand, anti-hypertensive
The Uses of Clonidine hydrochloride
In shaving soaps.
What are the applications of Application
Clonidine hydrochloride is an antihypertensive α2-adrenergic receptor agonist
Definition
ChEBI: Clonidine hydrochloride is a dichlorobenzene.
Manufacturing Process
N-(2,6-dichlorophenyl)thiourea (MP 149°C) was prepared in customary
manner from 2,6-dichloroaniline (Organic Synthesis III, 262-263) and
ammonium thiocyanate. 16.0 g of this thiourea derivative were refluxed for
2.5 hours together with 16 g of methyl iodide in 150 cc of methanol.
Thereafter, the methanol was evaporated out of the reaction mixture in vacuo,
leaving as a residue 22 g of N-(2,6-dichlorophenyl)-S-methyl-isothiouronium
hydroiodide of the formula having a melting point of 170°C. The entire residue
was then admixed with an excess (120%) above the molar equivalent of
ethylenediamine, and the mixture was heated for about one hour at 130° to
150°C. Methyl mercaptan was given off. Thereafter, the reaction mixture
comprising 2-(2',6'-dichloroanilino)-1,3-diazacyclopentene-(2) hydroiodide was
taken up in hot dilute acetic acid, and the resulting solution was made alkaline
with 2 N NaOH. A precipitate formed which was separated by vacuum
filtration, washed with water and dried. 4.0 g of 2-(2',6'-dichloroanilino)-1,3-
diazacyclopentene-(2) were obtained. The product had a melting point of
130°C.
The free base was then dissolved in absolute methanol, and the resulting
solution was then adjusted to an acid pH value with an ethereal hydrochloric
acid solution. The acidified solution was purified with charcoal and then dry
ether was added thereto until crystallization took place. The hydrochloride,
prepared in this customary manner, had a melting point of 305°C according to
US Patent 3,202,660
brand name
Catapres (Boehrin- ger Ingelheim); Duraclon (Xanodyne).
Therapeutic Function
Antihypertensive
Synthesis Reference(s)
Synthesis, p. 64, 1987 DOI: 10.1055/s-1987-27847
General Description
Clonidine hydrochloride, 2-[(2,6-dichlorophenyl)imino]imidazolidine monohydrochloride(Catapres), was the first antihypertensive known to acton the CNS. It was synthesized in 1962 as a derivativeof the known -sympathomimetic drugs naphazoline andtolazoline, potential nasal vasoconstrictors, but instead itproved to be effective in the treatment of mild-to-severe hypertension.Clonidine hydrochloride acts by both peripheral andcentral mechanisms in the body to affect blood pressure. Itstimulates the peripheral -adrenergic receptors to producevasoconstriction, resulting in a brief period of hypertension.
Biological Activity
Prototypical I 1 imidazoline receptor ligand. α 2 -adrenergic receptor agonist. Antihypertensive.
Biochem/physiol Actions
Clonidine hydrochloride is used for management of hypertension in pregnant women. In addition, it also acts as a therapeutic for neonatal abstinence syndrome. Clonidine hydrochloride binds to central α-adrenergic receptors and reduces the efferent sympathetic neuronal vasoconstrictor tone to the heart, kidneys and peripheral vasculature leading to vasodilatation and reduction in the blood pressure.
Clinical Use
Clonidine hydrochloride acts by both peripheral andcentral mechanisms in the body to affect blood pressure. Itstimulates the peripheral α-adrenergic receptors to producevasoconstriction, resulting in a brief period of hypertension.Clonidine hydrochloride acts centrally to inhibitthe sympathetic tone and cause hypotension that is ofmuch longer duration than the initial hypertensive effect.Administration of clonidine hydrochloride thus produces abiphasic change in blood pressure, beginning with a briefhypertensive effect and followed by a hypotensive effectthat persists for about 4 hours. This biphasic response isaltered by dose only. Larger doses produce a greater hypertensiveeffect and delay the onset of the hypotensiveproperties of the drug. Clonidine hydrochloride acts on 2-adrenoreceptors located in the hindbrain to produce itshypotensive action. Clonidine hydrochloride also acts centrallyto cause bradycardia and to reduce plasma levels ofrenin. Sensitization of baroreceptor pathways in the CNSappears to be responsible for the bradycardia transmittedby way of the vagus nerve. The central mechanism that resultsin decreased plasma renin is not known, however.The hypotensive properties of clonidine in animals can beblocked by applying -adrenergic blocking agents directlyto the brain.
Drug interactions
Potentially hazardous interactions with other drugs
Antidepressants: tricyclics antagonise hypotensive
effect and also increase risk of hypertension on
clonidine withdrawal; increased hypotensive
effect with MAOIs; hypotensive effect possibly
antagonised by mirtazapine.
Beta-adrenoreceptor antagonists: increased risk of
hypertension on withdrawal.
Ciclosporin: may increase ciclosporin levels.
Sympathomimetics: possibly increased risk of
hypertension with adrenaline and noradrenaline;
serious adverse effects reported with
methylphenidate.
Metabolism
About 50% of a of clonidine dose is metabolised in the
liver.
It is excreted in the urine as unchanged drug and
metabolites, 40-60% of an oral dose being excreted in 24
hours as unchanged drug; about 20% of a dose is excreted
in the faeces, probably via enterohepatic circulation.
storage
Store at RT
Purification Methods
This antihypertensive is recrystallised from EtOH/Et2O and dried in a vacuum (solubility in H2O is 5%). The free base has m 124-125o and is recrystallised from hexane. [Jen et al. J Med Chem 18 90 1975, NMR: Jackman & Jen J Am Chem Soc 97 2811 1975.]
Properties of Clonidine hydrochloride
| Melting point: | 312 °C |
| storage temp. | 2-8°C |
| solubility | H2O: 50 mg/mL, clear, colorless |
| form | solid |
| color | white |
| PH | pH(50g/l, 25℃) : 3.5~6.0 |
| Water Solubility | Soluble in water (50 mg/ml), DMSO (75 mM), methanol, chloroform (slightly), and dehydrated alcohol. |
| Merck | 14,2390 |
| BRN | 4163525 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 4205-91-8(CAS DataBase Reference) |
| EPA Substance Registry System | 1H-Imidazol-2-amine, N-(2,6-dichlorophenyl)-4,5-dihydro-, hydrochloride (1:1) (4205-91-8) |
Safety information for Clonidine hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P403+P233:Store in a well-ventilated place. Keep container tightly closed. |
Computed Descriptors for Clonidine hydrochloride
| InChIKey | GLEWMLFXCSBZLK-UHFFFAOYSA-N |
Clonidine hydrochloride manufacturer
Alembic Pharmaceuticals Limited
Ebenezer Industries
Alfa Omega Pharma
Shivalik Rasayan Ltd
Glyra Health Care Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 4205-91-8 Clonidine hydrochloride 98%View Details
4205-91-8 Clonidine hydrochloride 98%View Details
4205-91-8 -
 4205-91-8 98%View Details
4205-91-8 98%View Details
4205-91-8 -
 N-(2,6-Dichlorophenyl)-4,5-dihydro-1H-imidazol-2-amine HCl 98% CAS 4205-91-8View Details
N-(2,6-Dichlorophenyl)-4,5-dihydro-1H-imidazol-2-amine HCl 98% CAS 4205-91-8View Details
4205-91-8 -
 Clonidine hydrochloride 98%View Details
Clonidine hydrochloride 98%View Details
4205-91-8 -
 Clonidine hydrochloride 4205-91-8 98%View Details
Clonidine hydrochloride 4205-91-8 98%View Details
4205-91-8 -
 4205-91-8 Clonidine hydrochloride 98%View Details
4205-91-8 Clonidine hydrochloride 98%View Details
4205-91-8 -
 Clonidine Hydrochloride CAS 4205-91-8View Details
Clonidine Hydrochloride CAS 4205-91-8View Details
4205-91-8 -
 Clonidine hydrochloride CAS 4205-91-8View Details
Clonidine hydrochloride CAS 4205-91-8View Details
4205-91-8