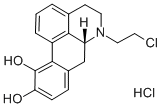
APOMORPHINE HYDROCHLORIDE
Synonym(s):R-(−)-Apomorphine hydrochloride hemihydrate
- CAS NO.:314-19-2
- Empirical Formula: C17H18ClNO2
- Molecular Weight: 303.78
- MDL number: MFCD00069236
- EINECS: 206-243-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
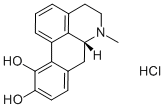
What is APOMORPHINE HYDROCHLORIDE?
Originator
Apomorphine hydrochloride,Nastech Pharmaceuticals Company, Inc.
The Uses of APOMORPHINE HYDROCHLORIDE
(R)-(-)-Apomorphine Hydrochloride is a prototypical dopamine agonist. Potential treatment for Parkinson’s disease.
The Uses of APOMORPHINE HYDROCHLORIDE
Emetic.
What are the applications of Application
(R)-(?)-Apomorphine hydrochloride is a prototypical dopamine agonist
Manufacturing Process
2 Methods of producing of apomorphine
1. The apomorphine was obtained by dehydratation of morphine at heating to
120°C in the presence phosphoric acid and rendering of HCl gas over reaction
mixture.
2. The morphine was converted to β-chloromorphine and then to
dichlorodihydrodesoxymorphine at heating to 140°-150°C in the presence
hydrochloric acid. Then apomorphine is obtained by dehydratation of
dichlorodihydrodesoxymorphine.
brand name
Apokyn (Vernalis).
Therapeutic Function
Emetic, Expectorant, Hypnotic, Antiparkinsonian, Dopamine agonist
General Description
Apomorphine hydrochloride,(6aR)-6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinolone-10,11-diol hydrochloride (Apokyn), is awhite or off-white powder or crystal soluble in hot water(pKa=8.92). Apormorphine is an aporphine alkaloid of thebenzoquinoline class. Oral apomorphine is poorly absorbedand has a bioavailability of less than 4%. Upon subcutaneousadministration, apomorphine is completely absorbed. Within10 to 20 minutes, the maximum concentration of the drug isdistributed from the blood plasma to the CSF. Other potentialroutes of administration include continuous subcutaneous infusion,intravenous infusion, intranasal spray application,sublingual, and rectal administration.23 The agent is highlylipophilic in nature, allowing for rapid diffusion across theBBB after injection. Apomorphine has a short plasma halflife;however, clinical effects may last from 60 to 90 minutes.Apomorphine displays a significant degree of interpatientvariability in its pharmacokinetic profile. Studies of bothintravenous and subcutaneous injection routes found this variation was not attributable to body weight, age, gender,and duration of PD or L-DOPA dose/duration alone.Apomorphine is extensively metabolized. Hypothesizedroutes include sulfation, N-demethylation, glucuronidation,and oxidation. Subcutaneous injections of apomorphine arerenally and hepatically cleared, with the majority appearingto be renally cleared. Dosage adjustments are needed in bothliver and renal impairment. The activity of apomorphine isbelieved to be caused by stimulation of postsynaptic D1- andD2-type receptors within the caudate/putamen in the brain.Apomorphine is indicated for the acute, intermittent treatmentof hypomobility, “off” episodes (“end-of-dose wearingoff” and unpredictable on/off episodes) associated with advancedPD.
Biological Activity
Prototypical dopamine agonist (pK i values are 6.43, 7.08, 7.59, 8.36 and 7.83 for human recombinant D 1 , D 2L , D 3 , D 4 and D 5 receptors respectively). Produces biphasic effects on locomotor activity, and displays anti-Parkinsonian and neuroprotective actions following systemic administration in vivo .
Clinical Use
Treatment of refractory motor fluctuations in Parkinson’s disease
Safety Profile
Poison by intravenous andintraperitoneal routes. Mutation data reported. When heated to decomposition itemits very toxic fumes of NOx and HCl.
Veterinary Drugs and Treatments
Apomorphine is used primarily as an emetic in dogs, and is considered the emetic of choice for dogs by many clinicians. It is sometimes used in cats, but its use in this species is somewhat controversial.
Drug interactions
Potentially hazardous interactions with other drugs
Antihypertensives: enhanced hypotensive effect.
Domperidone: possible increased risk of ventricular
arrhythmias.
5HT3
-receptor antagonists: possibly increased
hypotensive effects with ondansetron.
Nitrates: enhanced hypotensive effect.
Metabolism
After subcutaneous injection its fate can be described by a two-compartment model, with a distribution half-life of 5 (±1.1) minutes and an elimination half-life of 33 (±3.9) minutes. Clinical response correlates well with levels of apomorphine in the cerebrospinal fluid. Apomorphine is extensively metabolised in the liver, mainly by conjugation with glucuronic acid or sulfate; the major metabolite is apomorphine sulfate. It is also demethylated to produce norapomorphine. Most of a dose is excreted in urine, mainly as metabolites.
Storage
Store at RT
Properties of APOMORPHINE HYDROCHLORIDE
| Melting point: | >250℃ |
| storage temp. | Store at RT |
| solubility | ≥1.08 mg/mL in EtOH with ultrasonic; ≥12.9 mg/mL in DMSO; ≥5.12 mg/mL in H2O |
| form | solid |
| EPA Substance Registry System | Apomorphine hydrochloride (314-19-2) |
Safety information for APOMORPHINE HYDROCHLORIDE
Computed Descriptors for APOMORPHINE HYDROCHLORIDE
New Products
Sodium glycochenodeoxycholate, >98% Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Gabapentin EP Impurity A 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 1,3-Diphenylurea 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID N-METHYL INDAZOLE-3-CARBOXYLIC ACID Zinc Bis-glycinate 2-Hydroxy-5-nitroacetophenone 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylate 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2-HYDROXY BENZYL CYANIDE DIETHYL AMINOMALONATE HYDROCHLORIDE 5-BROMO-2CYANO PYRIDINE Boldenone Clarithromycin Ethinyl Estradiol StanozololRelated products of tetrahydrofuran


![(R)-6-ETHYL-5,6,6A,7-TETRAHYDRO-4H-DIBENZO[DE,G]QUINOLINE-10,11-DIOL HYDROCHLORIDE](https://img.chemicalbook.in/CAS/GIF/18426-16-9.gif)

You may like
-
 314-19-2 Apomorphine hydrochloride 98%View Details
314-19-2 Apomorphine hydrochloride 98%View Details
314-19-2 -
 CIS BROMO BENZOATE 98%View Details
CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 90-01-7 2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 3-NITRO 2-METHYL BENZOIC ACID 98%View Details
3-NITRO 2-METHYL BENZOIC ACID 98%View Details
1975-50-4 -
 INDAZOLE-3-CARBOXYLIC ACID 4498-67-3 98%View Details
INDAZOLE-3-CARBOXYLIC ACID 4498-67-3 98%View Details
4498-67-3 -
 221615-75-4 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 98%View Details
221615-75-4 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 98%View Details
221615-75-4 -
 2033-24-1 MELDRUMS ACID 98%View Details
2033-24-1 MELDRUMS ACID 98%View Details
2033-24-1 -
 42831-50-5 98%View Details
42831-50-5 98%View Details
42831-50-5
