ANTHRALIN
Synonym(s):1,8,9-Anthracenetriol;1,8-Dihydroxy-9(10H)-anthracenone;1,8-Dihydroxyanthrone;Anthralin;Dithranol
- CAS NO.:1143-38-0
- Empirical Formula: C14H10O3
- Molecular Weight: 226.23
- MDL number: MFCD00053409
- EINECS: 214-538-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
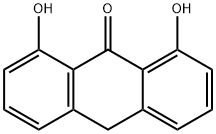
What is ANTHRALIN?
Absorption
Anthralin penetrates damaged skin and psoriatic lesions faster and to a greater extent than normal skin, likely due to increased vascularity of psoriatic lesions .
Toxicity
Some mild adverse effects include alterations in nail coloring, hair coloring, increase in photosensitivity, and skin irritation .
The most common side effects of anthralin are skin irritation and staining of nearby skin, nails, clothing, and other objects that come in contact with the treated patient. The incidence of irritation of psoriatic/surrounding healthy skin is higher in patients who leave anthralin on the skin without rinsing than in those who use short-contact therapy of 2 hours or less, followed by rinsing .
If the psoriatic plaques are well circumscribed, the surrounding normal skin may be protected by the use of a coating agent such as zinc oxide ointment. Anthralin should be applied cautiously to the face and intertriginous areas due to the risk of severe skin irritation .
There is no current evidence of any long-term anthralin toxicity related either to skin exposure or to systemic issues .
Some long-term studies in mice have demonstrated anthralin to be tumorigenic in mouse skin. This carcinogenic potential has not been thoroughly evaluated. Tumorigenic and carcinogenic effects of anthralin have not been observed in humans at this time . Anthralin is classified as FDA pregnancy risk category C drug . It is not known if anthralin can cause fetal harm when administered during gestation. Because of the lack of evidential human data, anthralin should be used during pregnancy only when clearly required .
Description
Anthralin is an anthrone inhibitor of keratinocyte proliferation and a modulator of differentiation. It increases apoptosis and inhibits proliferation of normal human keratinocytes (NHKs) when used at a concentration of 2.5 μM. It also decreases the mitochondrial membrane potential, increases cytochrome c release, and induces perinuclear mitochondrial clustering in NHKs when used at a concentration of 5 μM. Anthralin (0.25 μM) decreases the expression of β-defensin-2 and S100 calcium-binding protein A9 (S100A9) and increases the expression of IL-6 and IL-8 in IL-17A- and IL-22-stimulated NHKs. It also inhibits leukotriene B4 (LTB4; ) production, stimulated by the calcium ionophore A23187 , from human neutrophils (IC50 = 7 μM). Topical anthralin (0.1%) induces hair regrowth in a Dundee experimental bald rat (DEBR) model of alopecia areata.
Chemical properties
Yellow Crystalline Solid
The Uses of ANTHRALIN
Antipsoriatic
Background
Anthralin (1,8‐dihydroxy‐9anthrone, dithranol) is an older anti-psoriatic agent that was first synthesized as a derivative of chrysarobin, obtained from the araroba tree in Brazil over 100 years ago. Adverse effects of anthralin include irritation and discoloration of the skin .
This specific property of the molecule inspired workers to study details of its pharmacology. It is important to consider that the drug is relatively innocuous, yet effective, and systemic side effects have not been observed with this anthralin, in contrast to a wide variety of systemic and topical therapies for psoriasis .
Anthralin is also known as dithranol. It is a main active ingredient in topical skin formulations for the treatment of psoriasis. Various formulations of the drug are available, including solutions, foams, and shampoos . The chemical structure of anthralin allows for dual solubility, permitting the compound to be absorbed well through the epidermis .
Anthralin has also been studied in the treatment of warts, showing promising results . Salicylic acid is frequently added to anthralin to augment the stability of anthralin and to increase its penetration and efficacy .
What are the applications of Application
Anthralin is a leukotriene biosynthesis inhibitor
Indications
Stable plaque psoriasis of the skin and scalp .
It is also used topically in the management of psoriasis, dermatoses, and alopecia areata. Anthralin is also used in biomedical research due to its effect on EGFR autophosphorylation .
Definition
ChEBI: An anthracene compound derived by the substitution of -OH groups for hydrogen at C-1 and C-8, and with an oxo group at C-9.
Indications
Anthralin (Anthra-Derm) is a potent reducing agent whose mechanism of action is unknown. It is approved for the treatment of psoriasis and also may be helpful in alopecia areata. The major toxicities are discoloration of skin, hair, and nails and irritant dermatitis.
brand name
Anthra-Derm (Dermik); DrithoCreme (Dermik); Drithoscalp (Dermik); Lasan (Stiefel).
General Description
Lemon yellow leaflets or plates or an orange powder. Melting point 176-181°C. No odor or taste. Insoluble in water. Moderately toxic by ingestion, inhalation and skin absorption. Used as a treatment for psoriasis.
Air & Water Reactions
Unstable in air when in alkaline solution. Insoluble in water.
Reactivity Profile
ANTHRALIN reacts as a weak acid. Soluble in aqueous bases. May react with strong oxidizing agents. May be sensitive to light and moisture .
Hazard
Very irritating. Do not use on scalp or near eyes.
Fire Hazard
Flash point data are not available for ANTHRALIN; however, ANTHRALIN is probably combustible.
Pharmacokinetics
Anthralin is a natural anthraquinone derivative, anti-psoriatic and anti-inflammatory agent. It controls skin growth by reducing the synthesis of DNA and the mitotic activity in the hyperplastic epidermis, normalizing the rate of cell proliferation and keratinization .
Side Effects
Anthralin's side effects include pruritus, erythema, scaling, folliculitis, and lymphadenopathy. Staining of skin and clothes can also be an issue.
Metabolism
Anthralin is administered topically. Although the extent of systemic absorption after topical application has not been determined, no traces of anthraquinone metabolites were detected in the urine of treated subjects in a limited clinical study of anthralin cream , .
Anthralin does not inhibit hepatic microsomal enzyme activity .
Properties of ANTHRALIN
| Melting point: | 178-181 °C (lit.) |
| Boiling point: | 464.1±45.0 °C(Predicted) |
| Density | 1.419±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, soluble in methylene chloride, sparingly soluble in acetone, slightly soluble in ethanol (96 per cent). It dissolves in dilute solutions of alkali hydroxides. |
| form | neat |
| pka | 7.16±0.20(Predicted) |
| form | Solid |
| color | Yellow to Orange to Dark Yellow |
| Water Solubility | Insoluble in water. Soluble in acetic acid, chloroform (20 mg/mL), acetone, benzene, solutions of alkali hydroxides, fixed oil. Slightly soluble in 95% ethanol, alcohol, ether, and glacial acetic acid. |
| Merck | 14,684 |
| BRN | 2054360 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 1143-38-0(CAS DataBase Reference) |
| IARC | 3 (Vol. 13; Sup 7) 1987 |
| EPA Substance Registry System | Dithranol (1143-38-0) |
Safety information for ANTHRALIN
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for ANTHRALIN
| InChIKey | NUZWLKWWNNJHPT-UHFFFAOYSA-N |
ANTHRALIN manufacturer
Prachi Pharmaceuticals Pvt Ltd
Agon Pharma Private Limited
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
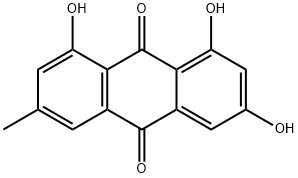
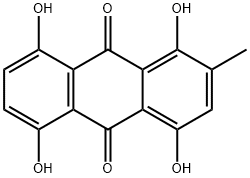
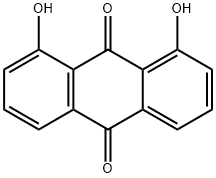

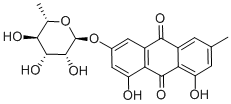
You may like
-
 1143-38-0 Dithranol 98%View Details
1143-38-0 Dithranol 98%View Details
1143-38-0 -
 Dithranol 99%View Details
Dithranol 99%View Details -
 Dithranol >98% (HPLC) CAS 1143-38-0View Details
Dithranol >98% (HPLC) CAS 1143-38-0View Details
1143-38-0 -
 Dithranol 98% (HPLC) CAS 1143-38-0View Details
Dithranol 98% (HPLC) CAS 1143-38-0View Details
1143-38-0 -
 Dithranol 99%View Details
Dithranol 99%View Details -
 Dithranol CAS 1143-38-0View Details
Dithranol CAS 1143-38-0View Details
1143-38-0 -
 Anthralin 90.00% CAS 1143-38-0View Details
Anthralin 90.00% CAS 1143-38-0View Details
1143-38-0 -
 Anthralin CAS 1143-38-0View Details
Anthralin CAS 1143-38-0View Details
1143-38-0