1,8-Dihydroxyanthraquinone
Synonym(s):1,8-Dihydroxyanthraquinone;Chrysazin, Dantron;Chrysazine;Danthron
- CAS NO.:117-10-2
- Empirical Formula: C14H8O4
- Molecular Weight: 240.21
- MDL number: MFCD00001211
- EINECS: 204-173-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
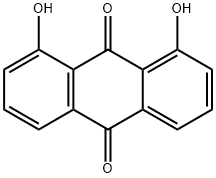
What is 1,8-Dihydroxyanthraquinone?
Description
Danthron, a natural product, was originally extracted from
the roots and rhizome of Polygonaceae plant, also
called Da Huang in traditional Chinese herbal medicine.
Now it is synthesized in many countries, such as Germany,
India, Japan, Poland, the United Kingdom, and the United
States. Danthron is reasonably anticipated to be a human
carcinogen.
Danthron is an anthraquinone that exists at room temperature
as a red or orange crystalline powder.It is practically
insoluble in water, but soluble in a variety of solvents
(acetone, chloroform, diethyl ether, ethanol) and alkaline
hydroxide solutions. The stability of danthron is generally
good. It is stable under room temperatures and normal pressures.
Chemical properties
orange-brown or brown powder
Chemical properties
Red-orange to orange crystalline powder or reddish-brown crystalline solid.
The Uses of 1,8-Dihydroxyanthraquinone
1,8-Dihydroxyanthraquinone can be used:
- To prepare an inclusion complex with β-cyclodextrin, applicable as a sensor in the estimation of Cu2+?ions in an aqueous solution.
- As a starting material in the synthesis of 1,4,5,8-tetramethoxyanthracene.
- As an aromatic scavenger in the modification of lignin, which acts as a corrosion inhibitor for steel.
The Uses of 1,8-Dihydroxyanthraquinone
Used as a stimulant laxative, though due to its carcinogenic properties, is not widely prescribed.
The Uses of 1,8-Dihydroxyanthraquinone
cathartic
The Uses of 1,8-Dihydroxyanthraquinone
Important intermediate in the manufacture of alizarin and indanthrene dyestuffs; forms insoluble Ca, Ba, Pb lakes. Antioxidant in synthetic lubricants; fungicide.
Background
Withdrawn from the Canadian, US, and UK markets in 1998 due to genotoxicity.
Definition
ChEBI: 1,8-Dihydroxyanthraquinone is a dihydroxyanthraquinone that is anthracene-9,10-dione substituted by hydroxy groups at positions 1 and 8. It has a role as an apoptosis inducer and a plant metabolite.
brand name
Dorbane (3M Pharmaceuticals); Istizin (Sterling Winthrop);Doss;Normax;Regulex-d.
World Health Organization (WHO)
Dantron, an anthroquinone derivative, has been available for over twenty years and is widely used as a laxative. The results of two chronic toxicity studies in rodents, published in 1985 and 1986, have shown that administration of high doses is associated with the development of intestinal and liver tumours.
General Description
Orange crystalline powder. Almost odorless and tasteless.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
1,8-Dihydroxyanthraquinone is incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides.
Fire Hazard
Flash point data for 1,8-Dihydroxyanthraquinone are not available; however, 1,8-Dihydroxyanthraquinone is probably combustible.
Side Effects
Liver injury may occur with 1,8-Dihydroxyanthraquinone when used as a laxative for one year. Symptoms disappeared after discontinuation of the drug, but recurred after resumption of the drug; none of the drugs administered alone had any effect on liver function test results. In addition, deep skin discolouration may occur with heavy use, mainly in elderly subjects, and is limited to the buttocks and thighs, with mild signs of inflammation. Skin contact with faeces or urine containing the drug appears to be a prerequisite for discolouration. Inflammation, if present, may be due to reduction of the parent compound in the colon to diol derivatives, which can irritate the intestine and skin in a way that the parent compound does not. There is also the possibility of colorectal melanosis.
Safety Profile
Confirmed carcinogen with experimental carcinogenic data. Moderately toxic by intraperitoneal route. An eye irritant. Questionable carcinogen with experimental carcinogenic and neoplastigenic data. Human mutation data reported. A laxative. When heated to decomposition it emits acrid smoke and irritating fumes.
Synthesis
1,8-Dihydroxyanthraquinone is prepared by replacing SO3H, by directly replacing nitro groups in sulfolane in the presence of calcium oxide , or, better, via 1,8-dimethoxyanthraquinone and subsequent hydrolysis of the ether .
Potential Exposure
A potential liver carcinogen and possible narcotic, this compound is no longer sold or marketed in the United States Nervous system toxin-acute effects; Respiratory toxin-acute effects other than severe or moderate irritation; Liver-acute effects; Eye irritant-mild.
Carcinogenicity
Danthron is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Environmental Fate
Danthron can cause DNA damage particularly at guanines in the 5'-GG-3', 5'-GGGG-3', 5'-GGGGG-3' sequences in the presence of Cu(II), cytochrome P450 reductase and the nicotinamide adenine dinucleotide phosphate (NADPH)-generating system. H2O2 and Cu(I) may also be involved because this DNA damage can be inhibited by catalase and bathocuproine. The further mechanism is danthron is reduced by P450 reductase and generate reactive oxygen species through the redox cycle, leading to extensive Cu(II)-mediated DNA damage. The DNA damage also comes from similar topoisomerase II inhibitor behavior of danthron.
Metabolism
Not Available
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
Crystallise Danthrone from EtOH and sublime it in a vacuum. [Beilstein 8 IV 3217.]
Toxicity evaluation
Danthron is discovered in several species of plants and insects.
It has been isolated from dried leaves and stems of Xyris semifuscata
harvested in Madagascar, and roots of Da Huang,
a Chinese traditional herbal medicine. Danthron also appears
to be biosynthesized by some insects. The presence of danthron
in insects may be a way of protection from predators. Danthron
can be manually synthesized by many countries. In the United
States, danthron was available from 12 suppliers.
If released to the atmosphere, danthron will exist in both
the vapor phase and the particulate phase. Vapor phase danthron
has an estimated half-life of 11 days. Particulate phase
danthron can be physically removed from air by wet and dry deposition. It is expected to biodegrade with 68% degradation
within 3 months.
If released to water, danthron is expected to adsorb to the
surface of solid particle and sediment. Biodegradation is also
a major pathway processed in water. It was reported that 82%
of the added danthron was degraded by fresh water within
3 days. If added to seawater, 91% of danthron was reported as
degraded. Danthron may bioconcentrate in aquatic organisms,
such as fish and shrimps.
Incompatibilities
Keep away from strong reducing agents, such as hydrides, nitrides, alkali metals, and sulfides.
Waste Disposal
It is inappropriate and possibly dangerous to the environment to dispose of expired or waste drugs and pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double- bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to prop- erly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.
Properties of 1,8-Dihydroxyanthraquinone
| Melting point: | 191-193 °C (lit.) |
| Boiling point: | 342.92°C (rough estimate) |
| Density | 1.3032 (rough estimate) |
| refractive index | 1.5430 (estimate) |
| Flash point: | >200°C |
| storage temp. | Sealed in dry,2-8°C |
| solubility | very faint turbidity in hot Acetic acid |
| form | Powder |
| pka | 6.27±0.20(Predicted) |
| color | Orange-brown or brown |
| Water Solubility | insoluble |
| Merck | 14,2815 |
| BRN | 2054727 |
| CAS DataBase Reference | 117-10-2(CAS DataBase Reference) |
| IARC | 2B (Vol. 50) 1990 |
| NIST Chemistry Reference | 1,8-Dihydroxyanthraquinone(117-10-2) |
| EPA Substance Registry System | Danthron (117-10-2) |
Safety information for 1,8-Dihydroxyanthraquinone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for 1,8-Dihydroxyanthraquinone
1,8-Dihydroxyanthraquinone manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 1,8-Dihydroxyanthraquinone CAS 117-10-2View Details
1,8-Dihydroxyanthraquinone CAS 117-10-2View Details
117-10-2 -
 Chrysazin CAS 117-10-2View Details
Chrysazin CAS 117-10-2View Details
117-10-2 -
 Danthrone CAS 117-10-2View Details
Danthrone CAS 117-10-2View Details
117-10-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1