Anisodine
- CAS NO.:52646-92-1
- Empirical Formula: C17H21NO5
- Molecular Weight: 319.35
- MDL number: MFCD00800686
- Update Date: 2024-10-23 13:36:13
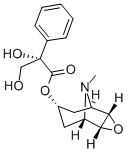
What is Anisodine?
Description
Anisodine is isolated from the roots of the plant Solanaceae Anisodus tanguticus (Maxim.) Pasch. (Scopolia tangutica Maxim.), which has a common name of Zhang-Liu-Shen, growing at the plateau with an altitude of 1700–4300?m and richly distributed in Tibet, Sichuan, Qinghai, Gansu, and other provinces in China.
Physical properties
Appearance: white crystals or crystalline powder. Flash point: 253.2?°C. Melting point: 126–128? °C (acetone-H2O), 190–192? °C (95% ethanol), and 197–200? °C (absolute ethanol). MS m/e (%): 319(51), 154(14), 138(100), 96(5), 95(5), 94(36), 97(15), 96(5), 42(15), 137(25), and 119(23).
History
Anisodine hydrobromide was an originally created new drug in China. It was separated from the herbal medicine Anisodus tanguticus from Qinghai province. In the early 1960s, Anisodus tanguticus was given the common name of Zhang-Liu-Shenin Qinghai province, similar to the common name Zhang Liu of Radix Phytolaccae. As a result, the root was misused as Radix Phytolaccae until the occurrence of atropine poisoning symptoms. Histological identification found that it is Anisodus tanguticus (Maxim.) Pasch, not Radix Phytolaccae. In order to make full use of the wild plant’s resources and develop the products of henbane drugs in China, the Chinese Academy of Medical Sciences carried out the systemic research on the plant .
Indications
Anisodine hydrobromide is recorded in the second volume of national standards for chemical drugs of the People’s Republic of China. The formulation consists of injection and tablet. It is used clinically for treatment of migraine, retinal vascular spasm, ischemic lesions, shrinking of the optic nerve, retina, and choroid, etc. It could improve the functional recovery after acute paralysis induced by inflammation of the nervous system and cerebrovascular disease, paralysis agitants, and carbon monoxideinduced toxic encephalopathy. It was also used for intravenous combined anesthesia, organophosphorus poisoning, bronchitis, asthma, and prevention and treatment of seasickness.
Pharmacology
Central anticholinergic effects: anisodine competes with acetylcholine in the M cholinergic receptor to prevent acetylcholine binding M cholinergic receptor, thus blocking the nerve impulse transmission and interfering with the physiological function based on the cholinergic neurotransmission. It has similar or slightly weaker effects than that of atropine on electrical activity of the brain, on pain caused by tremorine, and on tremor caused by arecoline in mice. Its effect is 19 times weaker than those of scopolamine . Peripheral anticholinergic effects: anisodine possesses antispasmodic and antiasthma effects and inhibits saliva secretion and mydriasis, which are weaker than those of atropine. It acts against organic phosphate pesticide poisoning. Anti-shock effect: anisodine prolonged significantly the survival time of animals or reduced mortality in patients with clinical shock through directly relieving vasospasm of vascular smooth muscle, antagonizing adrenaline-induced vasoconstriction, and improving microcirculation . Anti-cerebral ischemic disease: the effect of anisodine on cerebral blood circulation is mainly to regulate vasomotor, decrease cerebral vascular resistance, and increase cerebral blood flow, thereby improving the symptoms of cerebral ischemia.
Clinical Use
In the past, anisodine was mainly used in the treatment of various diseases of the central nervous system. It had a certain therapeutic effect on patients with very low vision and deservesclinical application . When used for ischemic optic neuropathy treatment, anisodine significantly promoted the recovery of the visual field, with the effective rate as high as 82.14% compared with conventional therapeutic regimen using corticosteroids combined with vasodilators, thrombolytic agents, vitamins, and antibiotics.
Properties of Anisodine
| Melting point: | 199.5-200 °C |
| Boiling point: | 495.0±45.0 °C(Predicted) |
| Density | 1.39±0.1 g/cm3(Predicted) |
| pka | 11.34±0.29(Predicted) |
Safety information for Anisodine
Computed Descriptors for Anisodine
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1