Anirolac
- CAS NO.:66635-85-6
- Empirical Formula: C16H15NO4
- Molecular Weight: 285.29
- MDL number: MFCD00866124
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
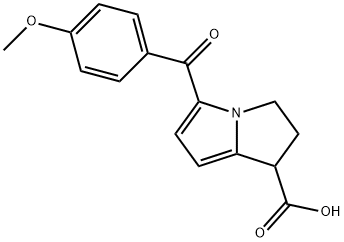
What is Anirolac?
Originator
Anirolac,Syntex Inc.
The Uses of Anirolac
Anirolac is a nonsteroidal anti-inflammatory drug with analgesic properties. Study suggests that pain relief due to Anirolac is equivalent to that of Naproxen (N377520).
The Uses of Anirolac
Antiinflammatory; analgesic.
Manufacturing Process
A solution of 1.1 equivalent of N,N-dimethyl-p-methoxybenzamide and 1 equivalent of phosphorous oxychloride in 2 ml of 1,2-dichloroethane is
refluxed for 30 minutes. To this solution is added a solution of 1 equivalent of
isopropyl 1,2-dihydro-3H-pyrrolo[1,2-a]pyrrole-1-carboxylate in 2 ml of 1,2-
dichloroethane. The reaction mixture is refluxed under an argon atmosphere
for 8 hours, treated with equivalent of sodium acetate and refluxed for a
further 5 hours. The resultant mixture is then evaporated to dryness and the
residue is chromatographed on 12 g of silica gel, eluting with hexane: ethyl
acetate (3:1), monitoring the course of the reaction by TLC. Isopropyl 5-pmethoxybenzoyl-
1,2-dihydro-3H-pyrrolo[1,2-a]pyrrole-1-carboxylate was
obtained as an oil. UV, IR, NMR spectrum confirmed the structure of obtained
compound.
A solution of 1 equivalent of isopropyl 5-p-methoxybenzoyl-1,2-dihydro-3Hpyrrolo[
1,2-a]pyrrole-1-carboxylate in 10 ml of methanol is treated with a
solution of 1 equivalent of potassium carbonate in 5 ml of water. The reaction
mixture is refluxed under nitrogen atmosphere for 30 minutes, cooled, and
evaporated to dryness. The residue is taken up in 10 ml of 10% aqueous
hydrochloric acid and 50 ml of water and the resultant mixture extracted with
ethyl acetate (2x50 ml). The combined extracts are dried over magnesium
sulfate and evaporated to dryness under reduced pressure. Crystallization of
the residue from ethyl acetate-hexane affords 5-p-methoxybenzoyl-1,2-
dihydro-3H-pyrrolo[1, 2-a]pyrrole-1-carboxylic acid (anirolac); MP: 187°-
187.5°C.
Therapeutic Function
Antiinflammatory, Analgesic
Properties of Anirolac
| Boiling point: | 532.6±50.0 °C(Predicted) |
| Density | 1.33±0.1 g/cm3(Predicted) |
| pka | 4.27±0.20(Predicted) |
Safety information for Anirolac
Computed Descriptors for Anirolac
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1