ANDROMEDOTOXIN
Synonym(s):Acetylandromedol;Andromedotoxin;Rhodotoxin
- CAS NO.:4720-09-6
- Empirical Formula: C22H36O7
- Molecular Weight: 412.52
- MDL number: MFCD29920140
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
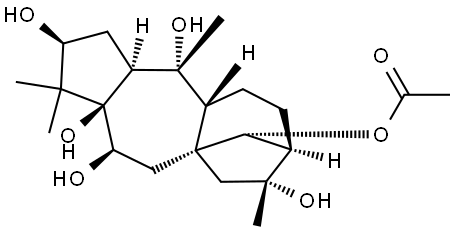
What is ANDROMEDOTOXIN?
Description
Fructus Rhododendri (ba li ma) is the dried fruit of Rhododendron molle (Bl.)
G. Don (yánɡ zhí zhú), mainly distributed in the Jiangsu, Hubei, and Jiangxi
Provinces of China .
“Compendium of Materia Medica” recorded that Rhododendron molle (Bl.)
G. Don (yánɡ zhí zhú) can be used to treat waist pain, arm pain, and poisoning. It is
effective at lowering blood pressure and slowing the heart rate, but a large dosage
can cause death . Rhomotoxin’s formulations include injection and tablet. Side
effects include a burning sensation, numbness, dizziness, dry mouth, nausea, vomiting,
vertigo, chest tightness, slow heartbeat, and low blood pressure. In addition,
pregnant women should use it with caution.
Physical properties
Appearance: white crystalline or powder. Solubility: soluble in water, acetone, and chloroform, almost insoluble in diethyl ether or petroleum ether. Melting point: 265–268 °C. Specific optical rotation: ?50 to ?60°.
History
Because rhomotoxin can slow down heart rhythm and blood pressure, Deng Dao ji
et al. from the First Affiliated Hospital of Wuhan Medical College researched
Fructus Rhododendri in the early 1970s. They extracted the crystallization from
fruits and obtained preliminary results by animal testing and clinical application .
Rhomotoxin is a white needle crystal that is extracted from the dried fruit of
Rhododendron molle (Bl.) G. Don (yánɡ zhí zhú). Its biological activity is closely
related to three-dimensional specificity and hydrophobicity. 5β-hydroxyl, 6β-
hydroxyl, and, especially, the 2,3-epoxy groups can affect its biological activity .
Rhomotoxin was acetylated to obtain monoacetate (Japanese Rhododendron II).
The melting points of rhomotoxin and Japanese Rhododendron III are identical
(284–286 °C). IR and Rf of silica gel thin layer chromatography are identical.
Therefore, rhomotoxin is Japanese Rhododendron III .
Definition
ChEBI: A tetracyclic diterpenoid that is grayanotoxane in which the pro-R hydrogen at position 14 is substituted by an acetoxy group and in which the 3beta-, 5-, 6beta-, 10-, and 16- positions are su stituted by hydroxy groups.
Pharmacology
The pharmacological effects of rhomotoxin are mainly displayed in three aspects:
(1) analgesic effect: the suspension, infusion, and alcohol made of rhomotoxin fruit
have an analgesic effect in mice ; (2) effects on cardiovascular system: the resting
heart rate is a bad prognostic indicator of hypertension and other cardiovascular
diseases. Rhomotoxin can reduce the resting heart rate; (3) kidney protection: rhomotoxin
promotes good blood pressure and a slow heart rate and provides kidney
protection. Its mechanism may be related to a reduction in the content of angiotensin
II (Ang II), increasing endothelial nitric oxide synthase (eNOS) content, and
slowing heart rate .
The plasma concentration of mice decreased rapidly over time following intravenous
injection of rhomotoxin; there was no residue after 6 h. Rhomotoxin is rapidly
distributed to various organs through the blood circulation and reaches a peak within
5–15 min. The gallbladder (including bile) contained the highest amount, followed
by the liver and kidneys. It affects, in order, the gallbladder (including bile), liver,
kidney, thyroid, stomach, adrenal gland, heart, lungs, and brain. Rhomotoxin is
excreted mainly by the kidney and digestive tract. More prototype drugs are in the
urine than in the stool. Rhomotoxin has a stimulating effect on the stomach. A certain
amount of rhomotoxin also entered the thyroid and adrenal glands. A very small
amount was found in brain tissue, demonstratinge that rhomotoxin does not easily
pass through the blood-brain barrier. Plasma dialysis showed that the plasma protein
binding rate of rhomotoxin can reach as high as 60% in 30 min .
Clinical Use
Rhomotoxin acts as an antihypertensive drug, and it is able to lower the blood pressure in patients with severe hypertension. Its pharmacological effects are related to parasympathetic function. Large-dosage-induced low blood pressure can be restored with ephedrine. Its antihypertensive effect lasts for 0.5–1 h. If blood pressure has not dropped to a satisfactory level, rhomotoxin must not have been added because an excessive drop in blood pressure would lead to shock. Oral administration with aluminum hydroxide can reduce rhomotoxin-induced stomach discomfort. Intramuscular injection can also cause local pain. In addition, procaine reduces its antihypertensive effect. Secondary hypertension and malignant hypertension patients should use it with caution, critical and dying patients should be disabled (including, for example, severe heart failure, cardiomyopathy, atrioventricular block, and severe ventricular arrhythmias).
Safety Profile
Poison by subcutaneous,parenteral, intravenous and intraperitoneal routes. Whenheated to decomposition it emits acrid smoke and fumes.
Properties of ANDROMEDOTOXIN
| Melting point: | 258-260 to 267-270° |
| Boiling point: | 451.94°C (rough estimate) |
| alpha | D25 -8.8° (c = 2.3 in ethanol) |
| Density | 1.1142 (rough estimate) |
| refractive index | 1.5300 (estimate) |
| pka | 13.72±0.70(Predicted) |
| color | Crystals from EtOAc/pentane |
Safety information for ANDROMEDOTOXIN
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P262:Do not get in eyes, on skin, or on clothing. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for ANDROMEDOTOXIN
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4