Andrographolide
Synonym(s):;ent-(3β,12E,14R)-3,14,19-Trihydroxy-8(17),12-labdadien-16,15-olide;Andro;Andrographis;Andrographolide - CAS 5508-58-7 - Calbiochem
- CAS NO.:5508-58-7
- Empirical Formula: C20H30O5
- Molecular Weight: 350.45
- MDL number: MFCD07778082
- EINECS: 226-852-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-19 17:28:17
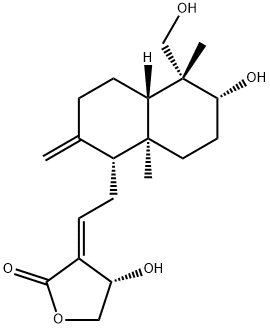
What is Andrographolide?
Description
Andrographolide is a diterpene lactone compound extracted from Andrographis.
paniculata (Burm. F) Nees. Andrographis paniculata is also known as lanhelian,
yijianxi, zhanshecao, kucao, kudancao, etc. Since this product originates in India, it
is also known as Indian grass. This product is dry ground part of Jupiteraceae plant
Andrographis paniculata (conus) may originate in South Asia. It is commonly cultivated in China such as in Fujian, Guangdong, Hainan, Guangxi, and Yunnan and
also in Australia and was also introduced in Jiangsu and Shaanxi.
Andrographis paniculata is commonly used in traditional Chinese medicine. Its
ability of relieving “snake venom, and internal injuries cough” was recorded in
“Lingnan herbal medicine records.” “Quanzhou Materia Medica” described it as
“heat-clearing and detoxifying, anti-inflammatory, detumescence and inhibition
throat inflammation, dysentery and high fever.”
The above ground part of Andrographis paniculata contains andrographolide,
neo-andrographolide, 14-deoxygenated andrographolide, etc., and the root contains
mainly flavonoids.
Chemical properties
white square prism or flaky crystals (ethanol or methanol) and odorless, with a bitter taste. dissolved in boiling ethanol, slightly soluble in methanol or ethanol, very slightly soluble in chloroform, and almost insoluble in water. Since andrographolide has an ester structure, it is easy to hydrolysis, open loop and isomerization in the aqueous solution to affect the drug stability. At lower temperature, the stability of andrographolide is better; it is unstable in alkaline conditions, and its instability increased with the increase in alkaline strength. The most stable pH value is 3–5. Andrographolide is more stable in chloroform.
Occurrence
Andrographis is found growing wild in India and Sri Lanka and is cultivated in many other parts of the world.
History
In 1911, Gorter firstly isolated a crystalline substance from Andrographis paniculata, identified it as a diterpene lactone compound, and named it andrographolide .
Andrographolide structure is complex and difficult to artificially synthesize, so it
is often extracted from the plant.
Andrographolide water solubility is poor and its bioavailability is low; its pharmacological effects are extensive but weak, so the preparation requirements are
strict. Therefore, from the 1970s, drugs and organic chemical researchers have done
a lot of work in the modification and transformation of andrographolide, mainly
concentrated in the α, β-unsaturated lactone double bond Michael addition, redox,
selective esterification of hydroxyl groups, oxidation and substitution reactions,
intramolecular cyclization, replacement of lactone rings, etc. They had obtained a
number of new derivatives. In the research of pharmacological effects, the main
study of the andrographolide derivatives focuses on antitumor, antivirus, and other
biological activities and has made some progress .
The Uses of Andrographolide
Andrographolide is a labdane diterpenoid. Andrographolide is the main bioactive component isolated from the medicinal plant Andrographis paniculata. It has a broad range of therapeutic applications including anti-inflammatory and anti-platelet aggregation activities and potential antineoplastic properties. Andrographolide showed significant antihepatotoxic action in P. berghei K173- induced hepatic damage in M. natalensis. Andrographolide inhibits tumor necrosis factor-α (TNF-α)-induced intercellular adhesion mol.-1 (ICAM-1) expression and adhesion of HL-60 cells onto human umbilical vein endothelial cells (HUVEC), which are associated with inflammatory diseases. These findings suggest that Andrographolide may have potential as a cardiovascular-protective agent.
What are the applications of Application
Andrographolide is an irreversible antagonist of NFκB and AP-1
Definition
ChEBI: Andrographolide is a labdane diterpenoid isolated from the leaves and roots of Andrographis paniculata that exhibits anti-HIV, anti-inflammatory and antineoplastic properties. It has a role as a metabolite, an anti-inflammatory drug, an anti-HIV agent and an antineoplastic agent. It is a gamma-lactone, a primary alcohol, a secondary alcohol, a labdane diterpenoid and a carbobicyclic compound.
What are the applications of Application
Andrographolide may be used as a reference standard for the determination of andrographolide in:
Leaves of Andrographis paniculata by soxhlet extraction-recrystallization-column chromatography purification followed by analyses using reversed phase high performance liquid chromatography (RP-HPLC) as well as gas chromatography-mass spectrometry (GC-MS).
Roots of Andrographis paniculata by HPLC with diode array detection (DAD), gas chromatography-mass spectrometry (GC-MS) and LC coupled to tandem mass spectrometry (MS/MS) equipped with electrospray ionization (ESI) and multiple reaction monitoring (MRM) mode of detection.
Stems of Andrographis paniculata by ultra-HPLC combined coupled to triple quadrupole-linear ion trap mass spectrometry (QqQLIT-ESI-MS/MS) with MRM detection.
Rat plasma samples by protein precipitation and subsequent ESI-LC-MS/MS determination using MRM detection.
General Description
Andrographolide, a bioactive diterpene lactone, is the main constituent of Andrographis paniculata, a plant used in traditional medicines. It is known to have different biological activities such as anti-inflammatory, anti-malarial, anti-cancer and hepatoprotective activity. It also shows potent anti-viral effect against dengue virus.
Biochem/physiol Actions
Diterpenoid lactone with anti-inflammatory properties. It blocks T-cell proliferation to allogenic stimuli and the chemotactic migration of macrophages induced by complement. Blocks the proliferation of several cancer cell lines in vitro.
Pharmacology
Modern pharmacological studies have shown that andrographolide has effects of
anti-inflammatory, antibacterial, antivirus, antitumor, and immune regulation and
can be used in treatment of cardiovascular-cerebrovascular diseases and protectionof the liver and gallbladder. Andrographolide can treat the fever caused by pneumococcus and hemolytic Streptococcus mutans, mainly by inhibiting the hypothalamus PGE2 and cAMP content to exert its antipyretic, nonetheless reducing the
chemotaxis (fMLP) CD11b + and CD18
+ may be the main mechanism of its antiinflammatory effect. Andrographolide has antagonistic effects on Hong Kong virus
(HKV), Ebola virus (EBOV), and respiratory syncytial virus (RSV) and has been
shown to inhibit HIV, SARS, and viral myocarditis ST2 in?vitro
Andrographolide can also ameliorate myocardial ischemia and serve as protection from ischemia-reperfusion injury. It also has protection effect of vascular endothelial cells as well as can regulate lipid disorder, lower blood pressure, and exert
effect of anti-atherosclerosis. It can also prevent angiogenesis after restenosis and
improve blood rheology. Its mechanisms are mainly due to its free radical scavenging and antiplatelet aggregation properties. Andrographolide can increase the bile
flow, bile salt, bile acid, and deoxycholic acid in experimental rats and guinea pigs
and can reverse the reduction of bile and cholic acid and other secretions caused by
paracetamol and improve liver function .
Anticancer Research
It is a labdane diterpenoid, which shows cytotoxic effect against different cancercell lines like human epidermoid cancer cells (KB), lymphocytic leukemia cells(P388), breast cancer cells (MCF-7), and colon cancer cells (HCT-116). It inhibitsthe proliferation of colon cancer cells (HT-29) and exerts pro-differentiative effecton mouse myeloid leukemia M1 cell line (Desai et al. 2008).
Clinical Use
This product is commonly used clinically for dysentery, leptospirosis, meningitis, pneumonia, upper respiratory tract infection, and enhancing adrenal cortical function. In view of the requirement for clinical viral infection emergency, it is important to introduce the hydrophilic group in different lactone structure, enhance its water solubility, and improve efficacy. There are a variety kinds of andrographolide injection, including potassium dehydroandrograpolide succinate needle, potassium sodium dehydroandrographolide succinate needle. Lianbizhi is the representative of this class of drugs in current clinical application. Oral administration of andrographolide may induce bitter vomiting. Oral administration of this product and other preparations in large doses can cause epigastric discomfort and loss of appetite. It had been reported of drug eruption, upper abdominal pain, and anaphylactic shock caused by intramuscular injection. Severe symptom response emerged such as chest tightness, shortness of breath, pallor, lip bruising, cold sweats, weak pulse, decreased blood pressure, etc. Light reactions include abdominal pain, vomiting, asthma, urticaria, pimples, dizziness, head swelling, sneezing, chest pain, etc. The reaction is real time and also 5–20?min after injection and gradually improves 5–45?min after rescue and individual recovered by 24?h. There are also reports of acute amniotic fluid blockage caused by weaning lotion exchange. In addition, taking into account the adverse effects of andrographolide on reproduction, it is recommended to be used cautiously by fertility couples and pregnant women.
in vitro
andrographolide was found to suppress the expression of inducible nitric oxide synthase in a concentration-dependent manner. in the andrographolide-treated group, a reduction of akt, c-jun n-terminal kinase and p65 phosphorylation was observed. andrographolide also caused a decrease in bcl-2/nf-κb expression and a dose-dependent increase in the expression of cleaved-caspase3/bax protein [1].
in vivo
rats were dosed with andrographolide intragastrically for 5 consecutive days in a hepatoprotection study. results indicated that andrographolide could up-regulate glutamate cysteine ligase catalytic and modifier subunits, heme oxygenase-1, superoxide dismutase-1, glutathione s-transferase protein and mrna expression in the heart, liver, and kidney [2].
References
1) Xia et al. (2004), Andrographolide attenuates inflammation by inhibition of NF-kappa B activation through covalent modification of reduced cysteine 62 of p50; J. Immunol., 173 4207 2) Chen et al. (2004), Andrographolide suppresses endothelial cell apoptosis via activation of phosphatidyl inositol-3-kinase/Akt pathway; Biochem. Pharmacol., 67 1337 3) Yu et al. (2003), Antihyperglycemic effect of andrographolide is streptozotocin-induced diabetic rats; Planta Med., 69 1075
Properties of Andrographolide
| Melting point: | 229-232 °C (lit.) |
| Boiling point: | 557.3±50.0 °C(Predicted) |
| alpha | -126 º (c=1.5,HAc) |
| Density | d421 1.2317 |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO (up to 25 mg/ml) or in Ethanol (up to 8 mg/ml). |
| form | Off-white solid |
| pka | 12.36±0.20(Predicted) |
| color | Off-white |
| optical activity | [α]20/D 126°, c = 1.5 in acetic acid |
| λmax | 223nm(MeOH)(lit.) |
| Merck | 14,633 |
| BRN | 42762 |
| Stability: | Stable for 2 years from date of purchsae as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 5508-58-7 |
Safety information for Andrographolide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Andrographolide
| InChIKey | BOJKULTULYSRAS-QPSYGYIJSA-N |
Andrographolide manufacturer
Kavya Pharma
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


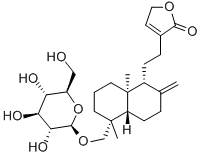
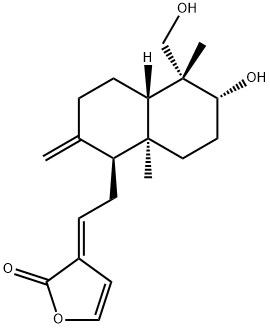
You may like
-
 Andrographolide 98%View Details
Andrographolide 98%View Details -
 Andrographolide 98% (HPLC) CAS 5508-58-7View Details
Andrographolide 98% (HPLC) CAS 5508-58-7View Details
5508-58-7 -
 Andrographolide 99% CAS 5508-58-7View Details
Andrographolide 99% CAS 5508-58-7View Details
5508-58-7 -
 Andrographolide 98% CAS 5508-58-7View Details
Andrographolide 98% CAS 5508-58-7View Details
5508-58-7 -
 ANDROGRAPHOLIDEView Details
ANDROGRAPHOLIDEView Details
5508-58-7 -
 Andrographolide Cas 5508 58 7View Details
Andrographolide Cas 5508 58 7View Details
5508-58-7 -
 Andrographolide CAS 5508-58-7View Details
Andrographolide CAS 5508-58-7View Details
5508-58-7 -
 Andrographolide 95%View Details
Andrographolide 95%View Details
5508-58-7
