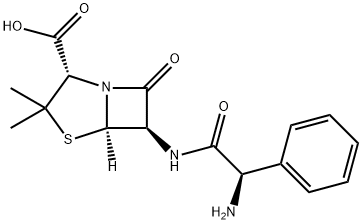
Ampicillin sodium
Synonym(s):Ampicillin sodium salt;Ampicillin, Sodium Salt - CAS 69-52-3 - Calbiochem;Ampicillin, Sodium Salt, Sterile, Tissue Culture Grade - CAS 69-52-3 - Calbiochem;Ampicillin, Sodium Salt, Sterile-Filtered Aqueous Solution, Cell Culture Tested - CAS 69-52-3 - Calbiochem;Xylose Lysine Deoxycholate Agar
- CAS NO.:69-52-3
- Empirical Formula: C16H20N3NaO4S
- Molecular Weight: 373.4
- MDL number: MFCD00064313
- EINECS: 200-708-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
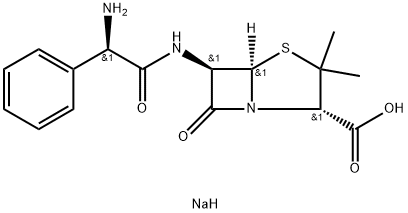
What is Ampicillin sodium?
Chemical properties
White or almost white powder, hygroscopic.
The Uses of Ampicillin sodium
Ampicillin sodium salt is a reagent for transformed cells expressing beta-lactamase. It acts as a structural analogue of acyl-D-alanyl-D-alanine, acylates the transpeptidase enzyme and prevents the cross-linking of the peptidoglycan of the cell wall necessary for the growth of the bacterium.
The Uses of Ampicillin sodium
Penicillin antibacterial.
The Uses of Ampicillin sodium
diuretic, anti-hypertensive
What are the applications of Application
Ampicillin sodium salt, cell culture grade is a widely used cell culture antibiotic
Definition
ChEBI: Ampicillin sodium is an organic sodium salt. It contains an ampicillin(1-).
brand name
Polycillin-N (Bristol-Myers Squibb).
General Description
Chemical structure: ?-lactam
Biological Activity
ampicillin sodium is a competitive inhibitor of the enzyme transpeptidase with ic50 value of 1.8 μg/ml [1].ampicillin is a β-lactam antibiotic that kills bacteria by inhibiting transpeptidase reactions. transpeptidase is involved in the final stages of cell wall biosynthesis and inhibition of it ultimately leads to cell lysis [1].in e. coli 146 cells treated with ether, ampicillin showed no significant inhibition on the transpeptidase reaction at concentrations below 0.5 μg/ml, but exhibited 50% inhibition at the concentration of 1.8 μg/ml. in e. coli 146 cells, the minimal inhibitory concentration (mic) of ampicillin was 3.1 μg/ml [1]. in e. coli and s. typhi, ampicillin and epicillin at concentrations close to mic values killed bacteria at slower rates than amoxycillin [2].in experimental mouse infections, oral or subcutaneous treatment of ampicillin at the dose of 0.2 ml/20 g was significantly more effective than epicillin and amoxycillin against the majority of infections [2].[1]. moore b a, jevons s, brammer k w. inhibition of transpeptidase activity in escherichia coli by thienamycin. antimicrobial agents and chemotherapy, 1979, 15(6): 831-833.[2]. basker m j, gwynn m n, white a r. comparative activities of ampicillin, epicillin and amoxycillin in vitro and in vivo. chemotherapy, 1979, 25(3): 170-180.
Contact allergens
Ampicillin caused contact dermatitis in a nurse also sensitized to amoxicillin (with tolerance to oral phenoxymethylpenicillin) and in a pharmaceutical factory worker. Systemic drug reactions are common. Crossreactivity is regular with ampicillin and can occur with other penicillins.
Biochem/physiol Actions
Mode of Action: This is a ?-lactam antibiotic that inhibits bacterial cell-wall synthesis by inactivating transpeptidases on the inner surface of the bacterial cell membrane. Mode of Resistance: Administration with ?-lactamase cleaves the ?-lactam ring of Ampicillin and inactivates it. Antimicrobial Spectrum: Includes both gram-positive (similar to benzylpenicillin) and gram-negative bacteria (similar to tetracyclines and chloramphenicol.
Safety Profile
Moderately toxic by intraperitoneal route. Human systemic effects by ingestion: hypermotility, diarrhea, and other gastrointestinal changes. Questionable carcinogen. When heated to decomposition it emits very toxic fumes of NOx, Na2O, and SOx.
Storage
+4°C
Properties of Ampicillin sodium
| Melting point: | 215 °C (dec.)(lit.) |
| alpha | +246~+272° |
| storage temp. | 2-8°C |
| solubility | H2O: 50 mg/mL, clear, very faintly yellow |
| form | powder |
| color | white with slight yellow cast |
| PH | pH(100g/l, 25℃):8.0~10.0 |
| Water Solubility | Freely soluble in water. Sparingly soluble in acetone |
| BRN | 4119211 |
| CAS DataBase Reference | 69-52-3(CAS DataBase Reference) |
Safety information for Ampicillin sodium
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H334:Sensitisation, respiratory |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P284:Wear respiratory protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Ampicillin sodium
| InChIKey | XCZQUQSOMVIRFX-AGYZSTNLNA-N |
| SMILES | C([C@H]1C(S[C@]2([H])[C@H](NC(=O)[C@@H](C3C=CC=CC=3)N)C(=O)N12)(C)C)(=O)O.[NaH] |&1:1,4,6,10,r| |
Ampicillin sodium manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Ampicillin Sodium Salt (AMP-Na) CAS 69-52-3View Details
Ampicillin Sodium Salt (AMP-Na) CAS 69-52-3View Details
69-52-3 -
 Ampicillin sodium salt CAS 69-52-3View Details
Ampicillin sodium salt CAS 69-52-3View Details
69-52-3 -
 Ampicillin sodium CAS 69-52-3View Details
Ampicillin sodium CAS 69-52-3View Details
69-52-3 -
 XLD Agar CASView Details
XLD Agar CASView Details -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
