Amobarbital
Synonym(s):AMAL
- CAS NO.:57-43-2
- Empirical Formula: C11H18N2O3
- Molecular Weight: 226.27
- MDL number: MFCD00057558
- EINECS: 200-330-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
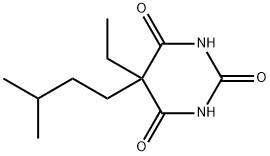
What is Amobarbital?
Chemical properties
Crystalline Solid
Originator
Hypnotal,Pharmacal
The Uses of Amobarbital
Amobarbital for binding at complex I to inhibit mitochondrial electron transport. Amobarbital is regulated as a schedule II compound in the United States and intended only for forensic and research purposes. This product is a qualified Reference Material (RM) that has been manufactured and tested to meet ISO17025 and Guide 34 guidelines. These materials are tested using validated analytical methods on qualified instrumentation to ensure traceability of measurements. All traceable RMs may be distinguished by their CofAs and can be downloaded below using the batch number located on the product label. For a representative CofA please contact our technical support.
The Uses of Amobarbital
Controlled substance (depressant). Sedative, hypnotic
The Uses of Amobarbital
Sedative, hypnotic. Controlled substance (depressant).
Background
A barbiturate with hypnotic and sedative properties (but not antianxiety). Adverse effects are mainly a consequence of dose-related CNS depression and the risk of dependence with continued use is high. (From Martindale, The Extra Pharmacopoeia, 30th ed, p565)
Definition
ChEBI: A member of the class of barbiturates that is pyrimidine-2,4,6(1H,3H,5H)-trione substituted by a 3-methylbutyl and an ethyl group at position 5. Amobarbital has been shown to exhibit sedative and hy notic properties.
Manufacturing Process
By interaction of malonic acid diethyl ester with sodium ethylate (molar ratio 1:1) and then with ethyl bromide (molar ratio 1:1) was prepared ethylmalonic acid diethyl ester. From ethylmalonic acid diethyl ester and sodium ethylate (molar ratio 1:1) and then with isopentylbromide was synthesized α-ethyl-α- isopentylmalonic acid diethyl ester. By condensation of α-ethyl-α- isopentylmalonic acid diethyl ester with urea in the presence of sodium ethylate was obtained ethyl-isopentylbarbituric acid.
brand name
Amytal Sodium (Lilly); Talamo (Marion Merrell Dow);Altinal;Alupent-sed;Ambese-la;Amobell;Amsal;Amycal;Amydorm;Amylbarb;Amylobeta;Analgilasa;Appenil;Asthmin;Beatol;Bludex;Calavon;Cuaot;Dexaspan;Dexital;Ergo-lonarid;Estimal;Etamyl;Ifenin;Isoamitil sedante;Isobec;Jalonac;Lonarid n;Medi-trol;Mudeka;Mylodorm sustrel;N 8;Neur-amyl;Novambobarb;Novogen;Obe_slim;Placidel;Protasma;Sedo-rythmodan;Sy-dexam;Transital.
Therapeutic Function
Hypnotic, Antiepileptic
World Health Organization (WHO)
Amobarbital is an intermediate-acting barbiturate which is controlled under Schedule III of the 1971 Convention on Psychotropic Substances. See WHO comment for barbiturates. (Reference: (UNCPS3) United Nations Convention on Psychotropic Substances (III), , , 1971)
General Description
White crystalline solid with no odor and a slightly bitter taste.
Air & Water Reactions
Amobarbital is hygroscopic . Insoluble in water.
Reactivity Profile
Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Hazard
May be a habit forming drug of abuse.
Fire Hazard
Flash point data for Amobarbital are not available, however, Amobarbital is probably combustible.
Safety Profile
A poison by ingestion,intravenous, intraperitoneal, and subcutaneous routes. Seealso BARBITURATES. When heated to decomposition itemits toxic fumes of NOx.
Metabolism
Not Available
Properties of Amobarbital
| Melting point: | 156-158°C |
| Boiling point: | 367.89°C (rough estimate) |
| Density | 1.1376 (rough estimate) |
| refractive index | 1.4620 (estimate) |
| Flash point: | 9℃ |
| storage temp. | -20°C |
| pka | 8.0(at 25℃) |
| color | Slightly bitter crystals or leaflets from water oralc |
| Water Solubility | <0.1 g/100 mL at 18.5 ºC |
| Stability: | Stable. Incompatible with strong oxidizing agents. Hygroscopic. |
| CAS DataBase Reference | 57-43-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Barbituric acid, 5-ethyl-5-isoamyl-(57-43-2) |
| EPA Substance Registry System | Amobarbital (57-43-2) |
Safety information for Amobarbital
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H336:Specific target organ toxicity,single exposure; Narcotic effects H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Amobarbital
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 AMAI human cell line CASView Details
AMAI human cell line CASView Details -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
