AMMONIUM CARBAMATE
Synonym(s):Ammonium carbamate;Ammonium carbaminate, Carbamic acid ammonium salt;Carbamic acid ammonium salt
- CAS NO.:1111-78-0
- Empirical Formula: CH6N2O2
- Molecular Weight: 78.07
- MDL number: MFCD00013010
- EINECS: 214-185-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
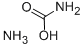
What is AMMONIUM CARBAMATE?
Chemical properties
crystal(s) powder(s); ammonia odor; white; rhomb; very volatile; forms urea when heated; made from liquid NH3 and solid CO2; used as a fertilizer [HAW93] [MER06]
Chemical properties
Ammonium carbamate is a colorless crystalline powder or white powder. Ammonia-like odor. The odor threshold is 5 ppm as NH3 (detection) and 46.8 ppm as NH3 (recognition).
The Uses of AMMONIUM CARBAMATE
Ammonium carbamate is used in the preparation of fertilizers. It acts as an ammoniating agent. It plays an important role in the formation of carbamoyl phosphate, which is essential for urea cycle and pyrimidines production. In addition to this, it is used in the preparation of trimethylsilyl carbamate.
The Uses of AMMONIUM CARBAMATE
Ammoniating agent, less vigorous than NH3.
What are the applications of Application
Ammonium carbamate is necessary for both the urea cycle and the production of pyrimidines
General Description
A white crystalline solid used as a fertilizer. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment.
Air & Water Reactions
Water soluble.
Reactivity Profile
AMMONIUM CARBAMATE is a carbamate salt. Carbamates are chemically similar to, but more reactive than amides. Like amides they form polymers such as polyurethane resins. Carbamates are incompatible with strong acids and bases, and especially incompatible with strong reducing agents such as hydrides. Flammable gaseous hydrogen is produced by the combination of active metals or nitrides with carbamates. Strongly oxidizing acids, peroxides, and hydroperoxides are incompatible with carbamates. AMMONIUM CARBAMATE is unstable in an alkaline media . AMMONIUM CARBAMATE is incompatible with the following: Strong oxidizers, strongly alkaline pesticides . Moderate fire and explosion hazards when exposed to heat or flame [USCG, 1999].
Hazard
Evolves irritating fumes when heated.
Health Hazard
INHALATION: Irritating to mucous membranes of respiratory tract. EYES: Irritating.
Fire Hazard
Behavior in Fire: Moderate fire and explosion hazards when exposed to heat or flame
Safety Profile
Poison by intravenous route. See also CARBAMATES
Potential Exposure
It is used as a fertilizer and ammoniating agent.
Shipping
UN2757 Carbamate pesticides, solid, toxic,
Incompatibilities
Strong bases, strong oxidizers. Keep away from heat (forms urea), moisture, and direct sunlight.
Properties of AMMONIUM CARBAMATE
| Melting point: | 59-61°C (subl.) |
| Boiling point: | 58.76°C (estimate) |
| Density | 1,6 g/cm3 |
| vapor pressure | 100 mm Hg ( 26.7 °C) |
| refractive index | 1.4616 (estimate) |
| storage temp. | 2-8°C |
| solubility | 790g/l |
| form | Powder |
| color | White |
| PH | 10 (100g/l, H2O, 20℃) |
| explosive limit | 2% |
| Water Solubility | Soluble in cold water and ammonium hydroxide. Slightly soluble in alcohol. Insoluble in acetone. |
| Merck | 14,507 |
| BRN | 3914091 |
| Stability: | Stable, but may be moisture sensitive. Incompatible with strong oxidizing agents, strong acids, strong bases. |
| CAS DataBase Reference | 1111-78-0(CAS DataBase Reference) |
| EPA Substance Registry System | Ammonium carbamate (1111-78-0) |
Safety information for AMMONIUM CARBAMATE
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for AMMONIUM CARBAMATE
| InChIKey | BVCZEBOGSOYJJT-UHFFFAOYSA-N |
AMMONIUM CARBAMATE manufacturer
Vinipul Chemicals Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 1111-78-0 Ammonium carbamate 99%View Details
1111-78-0 Ammonium carbamate 99%View Details
1111-78-0 -
 1111-78-0 99%View Details
1111-78-0 99%View Details
1111-78-0 -
 Ammonium carbamate CAS 1111-78-0View Details
Ammonium carbamate CAS 1111-78-0View Details
1111-78-0 -
 Ammonium carbamate CAS 1111-78-0View Details
Ammonium carbamate CAS 1111-78-0View Details
1111-78-0 -
 Ammonium Carbamate CAS 1111-78-0View Details
Ammonium Carbamate CAS 1111-78-0View Details
1111-78-0 -
 Ammonium carbamate CAS 1111-78-0View Details
Ammonium carbamate CAS 1111-78-0View Details
1111-78-0 -
 Ammonium carbamate 95% CAS 1111-78-0View Details
Ammonium carbamate 95% CAS 1111-78-0View Details
1111-78-0 -
 Ammonium carbamate CAS 1111-78-0View Details
Ammonium carbamate CAS 1111-78-0View Details
1111-78-0