Amlexanox
Synonym(s):2-Amino-7-(1-methylethyl)-5-oxo-5H-[1]Benzopyrano[2,3-b]pyridine-3-carboxylic acid;AA 673;Amoxanox;CHX 3673
- CAS NO.:68302-57-8
- Empirical Formula: C16H14N2O4
- Molecular Weight: 298.29
- MDL number: MFCD00864790
- EINECS: 804-135-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
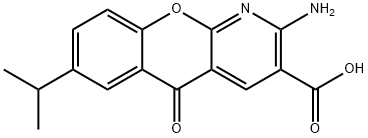
What is Amlexanox?
Absorption
No significant absorption directly through the active ulcer. Most of the systemic absorption is via the gastrointestinal tract.
Description
Amlexanox is an orally-active antiasthmatic agent useful in the treatment of bronchial asthma and allergy-related sinus disorders. The therapeutic effect of amlexanox appears to be attributed to its inhibitory actions on leukohiene D4, PAF and histamine.
Description
Amlexanox, sold under the trade name Aphthasol, is an anti-inflammatory drug that is used to treat canker sores, asthma, and other conditions. It was first patented for use against canker sores in 1994.
Recently, A. R. Saltiel and co-workers at the University of Michigan (Ann Arbor) found that amlexanox is a promising treatment for type 2 diabetes and obesity. They showed that it inhibits two kinases in mice that are produced in the liver and fat in response to inflammation caused by a high-fat diet. Obese mice treated with amlexanox lost weight and became less insulin resistant.
Chemical properties
White Crystalline Solid
Originator
Takeda (Japan)
The Uses of Amlexanox
Inhibits release of allergic mediators from mast cells. Antiallergic; antiasthmatic
Background
Amlexanox is an antiallergic drug, clinically effective for atopic diseases, especially allergic asthma and rhinitis. Amlexanox as a topical paste is a well tolerated treatment of recurrent aphthous ulcers. Recurrent aphthous ulcer (RAU) is the most prevalent oral mucosal disease in humans, estimated to affect between 5% and 50% of the general population.
What are the applications of Application
Amlexanox is an inhibitor of mast cell release of allergic mediators
Indications
Used as a paste in the mouth to treat aphthous ulcers (canker sores).
Definition
ChEBI: A pyridochromene-derived monocarboxylic acid having an amino substituent at the 2-position, an oxo substituent at the 5-position and an isopropyl substituent at the 7-position.
Manufacturing Process
A mixture of 2 ml of morpholine, 3 ml of dimethylformamide and 10 ml of water was heated to 60°C and under stirring the equal molecular quantity of 6-isopropyl-4-oxo-4H-1-benzopyran-3-carbonitrile was added for 5 minutes. The mixture was heated at that temperature for one hour and the resultant precipitate was filtered, rained with water recrystallized from acetic acid and washed with chloroform. By the above procedure was obtained 2-amino-6- isopropyl-4-oxo-4H-1-benzopyran-3-carboxaldehyde melting at 206°-208°C. A mixture of 4 ml ethyl cyanoacetate, 50 ml of ethanol, 5 ml of piperidine and the equal molecular quantity of 2-amino-6-isopropyl-4-oxo-4H-1-benzopyran- 3-carboxaldehyde was refluxed for 30 minutes and, after cooling, the crystalline precipitate was filtered and washed with chloroform. By above procedure was obtained ethyl-2-amino-7-isopropyl-1-azaxanthone-3- carboxylate, melting after recrystallization from ethanol at 243°-244°C. A mixture of 10 ml of acetic acid and 10 ml of 55% sulfuric acid the equal molecular quantity and 2-ethyl-amino-7-isopropyl-1-azaxanthone-3- carboxylate was stirred at 130°C for 4 hours and, after water was added, the precipitate was collected by filtration and recrystalllized from dimethylformamide to give the 2-amino-7-(1-methylethyl)-5-oxo-5H- [1]benzopyrano[2,3-b]pyridine-3-carboxylic acid, melting point 300°C.
brand name
Aphthasol (Uluru);Solfa.
Therapeutic Function
Antiulcer (topical)
General Description
Amlexanox is an anti-allergic drug with anti-inflammatory properties.
Biochem/physiol Actions
Amlexanox elevates the amount of nonsense-containing mRNAs in treated cells and helps to generate full-length proteins effectively.
Pharmacokinetics
Amlexanox is a mucoadhesive oral paste which has been clinically proven to abort the onset, accelerate healing and resolve the pain of aphthous ulcers (canker sores). It decreases the time ulcers take to heal. Because amlexanox decreases the healing time, it also decreases the pain you feel. Recent studies have also shown that the majority of ulcers can be prevented by application of the paste during the prodromal (pre-ulcerative) phase of the disease. Recurrent Aphthous Ulcers (RAU) also known as Recurrent Aphthous Stomatitis (RAS) is recognized as the most common oral mucosal disease known to man. Estimates suggest that 20% - 25% of the general population suffer at least one incidence of aphthous ulcers each year. Amlexanox is also being investigated for its anti-allergenic and anti-inflammatory properties.
Metabolism
Metabolized to hydroxylated and conjugated metabolites.
storage
Store at -20°C
References
1) Koch et al. (2013), Obesity: Teaching an old drug new tricks – amlexanox targets inflammation to improve metabolic dysfunction; Nat. Rev. Endocrinol., 9 185 2) Reilly et al. (2013), An inhibitor of the protein kinases TBK1 and IKK-ε improves obesity-related metabolic dysfunctions in mice; Nat. Med., 19 313 3) Nasry et al. (2016), Different modalities for treatment of recurrent aphthous stromatitis. A Randomized clinical trial; J. Clin. Exp. Dent. 8 e517 4) Cheng et al. (2018), Aphthous ulcer drug inhibits prostate tumor metastasis by targeting IKKε/TBK1/NFκB signaling; Theranostics 8 4633
Properties of Amlexanox
| Melting point: | >3000C |
| Boiling point: | 570.0±50.0 °C(Predicted) |
| Density | 1.408±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: soluble10mg/mL at warmed, clear |
| form | powder |
| pka | 3.95±0.20(Predicted) |
| color | white to beige |
| Merck | 14,490 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 3 months. |
| CAS DataBase Reference | 68302-57-8(CAS DataBase Reference) |
Safety information for Amlexanox
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Amlexanox
Amlexanox manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
![68302-57-8 2-Amino-7-isopropyl-5-oxo-5H- chromeno[2,3-b]pyridine-3-carboxylic acid 99%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/d402cbcb-0df1-4ea4-963e-a2a00bc9d609.png) 68302-57-8 2-Amino-7-isopropyl-5-oxo-5H- chromeno[2,3-b]pyridine-3-carboxylic acid 99%View Details
68302-57-8 2-Amino-7-isopropyl-5-oxo-5H- chromeno[2,3-b]pyridine-3-carboxylic acid 99%View Details
68302-57-8 -
 68302-57-8 98%View Details
68302-57-8 98%View Details
68302-57-8 -
 Amlexanox CAS 68302-57-8View Details
Amlexanox CAS 68302-57-8View Details
68302-57-8 -
 Amlexanox 98% CAS 68302-57-8View Details
Amlexanox 98% CAS 68302-57-8View Details
68302-57-8 -
 Amlexanox CAS 68302-57-8View Details
Amlexanox CAS 68302-57-8View Details
68302-57-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1